
|
by Larry P. Taylor, Ph. D.
Feedback appreciated; please send comments to: Email: lpt This site is best viewed with a screen resolution of 1280 x 1024. |
My University Home Harris Links Chemistry / Modeling Links
Modeling
Chemistry is defined as the study of matter and its interactions. Chemical reactions occur when atoms or molecules approach each other (Collision Theory) and combine to form new chemical species. These chemical processes can be better understood when reactants and products can be visualized. The purpose of molecular modeling is to provide a three-dimensional image (either physical or software-based) that allows a chemist to better see the manner in which atoms and molecules can interact. These models can be used to interpret existing observations or to predict new chemical behavior.
A fundamental approach to understanding our environment, at all levels from atomic to galactic, is that form (shape or architecture) and function are intimately related. Thus, an understanding of shape provides a clue as to function. An elementary view of this principle (a presentation for the Ann Arbor Hands-On Museum) can be found at Molecular Shape Determines Biological Activity.
Historically, atoms have been represented by spheres with their chemical bonds represented as springs or rods. These physical models can be useful in understanding the geometry of simple chemical structures. In particular, models are extremely helpful in organic or biochemistry because these disciplines study chemistry that has a true three-dimensional component. Physical models, particularly of proteins and DNA structures have allowed major strides in our understanding of biological activity. But, physical models of large molecules are expensive to construct, physically fragile, and difficult to alter or manipulate. For example, below is a model of a dopamine D2 receptor that represents about 5000 atoms. The 3' x 2' x 5' model needs to be supported by several aluminum rods; it took several weeks to assemble. This model was useful in predicting areas of the dopamine D2 receptor that might bind to anti-psychotic drugs involved in the treatment of mental disorders. Since rotating a molecule on a computer screen to look for areas of potential interest is a bit easier than turning a large physical assembly of rods, tubes and spheres, the study of large molecules is now best done with computer programs because such applications allow ease of viewing true three-dimensional molecular images. In addition, computer programs typically have numerous coloring schemes that allow the molecule under study to be colored by a variety of chemical or physical properties. This selective/easily changed coloring provides a powerful analysis tool that is missing in physical models.

The Dopamine D2 Receptor
The advent of computer modeling has totally changed the way chemistry is now done:
drugs are pre-screened in the computer prior to actual synthesis at a savings of millions of dollars per new drug
genetic diseases are becoming better understood as biochemical pathways are becoming better defined
hazardous interactions can be studied in the computer with little physical risk
One of the best freeware programs for teaching chemical structure is called MAGE. This program uses a format called Kinemages (Kinetic Images ... The Kinemage Home Page provides applications, utilities, databases, kinemage collections and tutorials for this protein structure rendering tool.). Although this application is primarily used in protein structure classes, web sites for sharing chemical information, and journal articles for emphasizing certain aspects of protein structure, it can also be used for simpler structures.
You can download standalone KiNG application for different platforms at KiNG DownloadIndex
MAGE is also available as a Java (computer protocol that allows web-based programs to run on most computers) application, KiNG (Kinemages Next Generation). This page uses KiNG to allow real-time user manipulation of a few selected molecules.
The real-time browser manipulation of molecules with KiNG requires a java-enabled (JRE from Java) browser.
Finally, while KiNG is a good application for
rendering geometry, it typically does not provide models that display either multiple
bond information or so-called space-filling rendering. So, Arguslab
was used to generate images showing
multiple bonds and Rastop
was used to create the ball-and-stick, as well as the space-filling images.
Rendering
Molecules are typically rendered on a computer screen in several different modes, depending on software/computer power and the needs of the user. Some useful forms:
Wireframe - bonds are depicted as thin lines with coloring indicating the atoms that are involved. The atom suggested by the color is at the end of the line representing the chemical bond. The color scheme (against a black background) is typically
Carbon = white or gray
Nitrogen = blue
Oxygen = red
Sulfur = yellow
Halides = light green
Hydrogen = white or cyan
Rods or sticks - the same as wireframe, except the diameter of the line connecting atoms is much larger. Since wireframe typically produces a poor quality captured or photographic image, rod format is sometimes considered a "photographic quality" wireframe rendering.
Ball & Stick - atoms are represented as small spheres and chemical bonds as rigid rods between them. The coloring scheme is the same as for wireframe.
Spacefilling - molecules are represented as spheres whose size is representative of the the atom's van der Waal's radius. The coloring is the same as wireframe.
For Water (H2O)
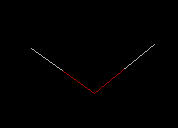 |
 |
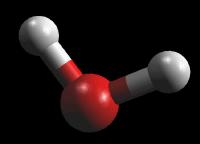 |
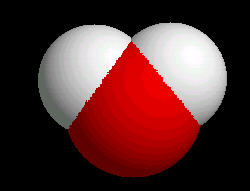 |
| Wireframe | Rods | Ball & Stick | Spacefilling |
Typically, a chemist works in wireframe (less image per molecule rendered) because computers generate wireframe images much faster than other formats. Spacefilling, because of the increased information displayed on the screen, is the slowest rendered image.
Ammonia
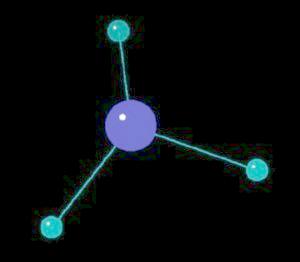
Ammonia (NH3) is typically found as a gas possessing a characteristic pungent odor. Ii is commonly found in household cleaning products. Although a mild base, it can be hazardous if spilled or inhaled. Ammonia is a primary source of biological nitrogen and is the first step in biosynthesis reactions that ultimately lead to proteins, vitamins, DNA and RNA type molecules. Commercially, it is used in preparing fertilizers, explosives and pharmaceuticals. Almost all nitrogen atoms used in biological processes or commercial chemical manufacturing comes form ammonia.
The ammonia molecule has a trigonal pyramidal shape. The nitrogen atom has an unshared pair of electrons that project away from the central nitrogen atom. This unshared pair (a proton acceptor) is what makes ammonia a base. The non-symmetrical shape of the ammonia molecule creates a dipole moment that makes the ammonia molecule polar. This polarity assists in creating a network of hydrogen bonds; an explanation for ammonia dissolving in water.
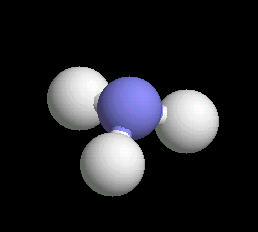 |
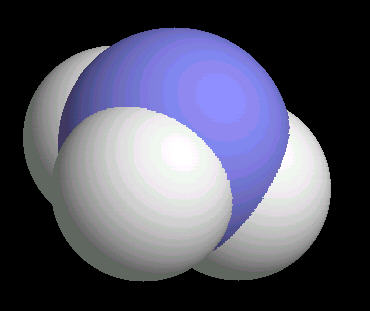 |
| Ammonia: Ball & Stick | Ammonia: Spacefilling |
Carbon Dioxide
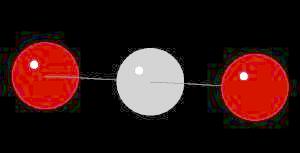
Carbon Dioxide (CO2) is a gas that averages ~380 ppm by volume in the earth's atmosphere. It is produced by biological respiration, burning of fossil fuels, and geothermal events such as volcanoes, hot springs and oceanic thermal vents. It is one of the major gasses involved in the greenhouse effect. As a solid ("dry ice") carbon dioxide sublimes (directly converts from solid to gaseous state) above -78 oC.
The carbon dioxide molecule has a linear shape determined by two double bonds (O=C=O) between the center carbon atom and each oxygen atom.
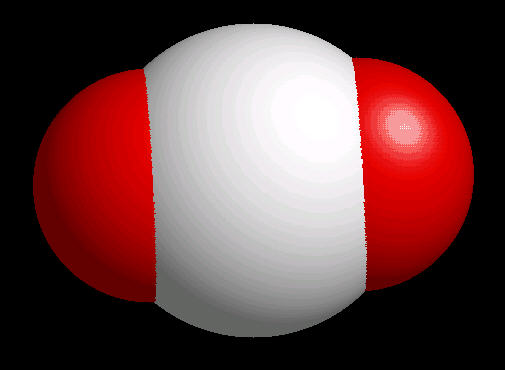 |
|
| Carbon Dioxide: Ball & Stick | Carbon Dioxide: Spacefilling |
Carbon Monoxide
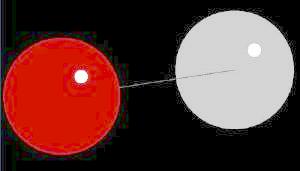
Carbon Monoxide (CO) is a colorless, odorless, tasteless and toxic gas. It binds to hemoglobin (the blood's oxygen carrying molecule) about 200 times stronger than oxygen. Since the presence of CO rapidly diminishes the body's ability to provide oxygen to tissues, its toxicity is related to oxygen starvation. Carbon monoxide is primarily produced by the incomplete combustion of fossil fuels. There is also some CO produced by animals as a product of bilerubin digestion. (Normally, not of concern, but the CO produced by this process can be lethal in closed environments),
When oxygen concentration is low, carbon monoxide forms in preference to carbon dioxide. Carbon monoxide is significant starting material for many commercial synthetic processes. Historically, passing water over heated coke produced a mixture of hydrogen and carbon monoxide called "water gas." (H2O + C => H2 + CO), a commercial fuel.
Carbon monoxide is a linear molecule showing a small dipole moment which is consistent with "partial" triple bond character between the carbon and oxygen atoms. As such, this molecule is best represented as a "resonance structure" of three primary forms: C- = O+ <=> C=O <=> C+ _ O-
|
|
|
| Carbon Monoxide: Ball & Stick | Carbon Monoxide: Spacefilling |
Ice
Ice (H2O)n Ice is hydrogen-bonded network of water.
Water is formed when two atoms of hydrogen bond covalently with an atom of oxygen. The molecule is not linear because the non-bonded electrons (2 unshared pairs) of oxygen repel each other; this leads to a non-linear (or bent-angular) structure.

Water Molecule
This bent water structure creates an equal distribution of electron density which creates a strong polarization of the water molecule The water molecule acts as if the hydrogen atoms were carrying a partial positive charge and the oxygen atom acts as if it were carrying a partial negative charge.
Water is called the universal solvent since it dissolves an amazing number of substances. In solution, water molecules surround the solute with the negative portion (oxygen atom) of the water molecule oriented towards the positive cation or positive portion of a polar solute; the positive hydrogen is oriented towards the anion or negative portion of a solute.
The weak interaction between the hydrogen atom of water and any more electronegative element (especially N, S, or F) is called a "hydrogen bond." While the individual hydrogen bond is relatively weak (compared to a covalent bond), large networks of hydrogen bonding can make significant contributions to the energy required to change physical state. This large network of hydrogen bonding explains the large amounts of energy required to alter the physical state of water because the large network of hydrogen bonds must be broken as part of the change-of-state process.
In ice, this network of hydrogen bonds (Hydrogen atoms oriented towards adjacent oxygen atoms) leads to a large (on the atomic scale) space between water molecules ... more space in the solid material (ice) than in liquid water. Since ice (at the atomic level) contains more space than an equivalent volume of liquid water, it is less dense than water and floats. This is an extremely important property. If ice were not less dense than water, then ice would not float and all fresh water bodies of water would freeze solid during the winter. This would terminate fresh water life.
|
|
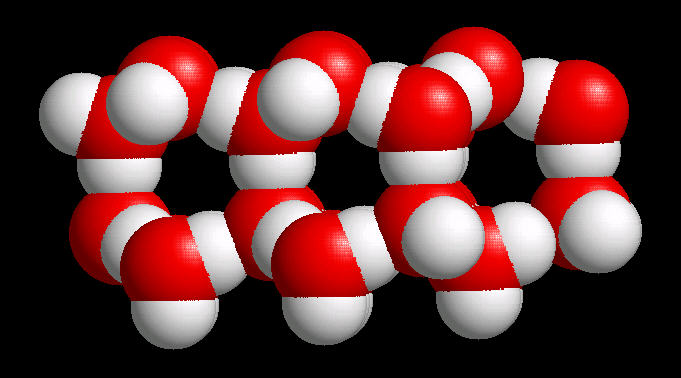 |
| Water Ice: Ball & Stick |
Water Ice: Spacefilling |
Sodium Chloride
Sodium Chloride (NaCl) is common table salt or halite. Salt is essential for life. In ancient times, wars were fought for control of salt resources since salt was more valuable than gold. Our term, salary, is based on the fact that Roman soldiers were paid in salt. Commercially, the electrolysis of salt is used to produce chlorine, hydrogen, and sodium hydroxide (2 NaCl + 2 H2O → Cl2 + H2 + 2 NaOH). Salt is also used as a starting material to synthesize sodium metal, sodium carbonate, calcium carbonate, sodium sulfate and hydrochloric acid.
Sodium chloride forms cubic crystals. The Sodium Chloride Unit Cell (each ion is surrounded by 6 of its opposite electrostatic charge) of sodium ions with the larger chloride ions is described as cubic close packing, octahedral symmetry, or the halite structure. Many other minerals form this type of crystal. The crystal structure is held together by electrostatic charges.
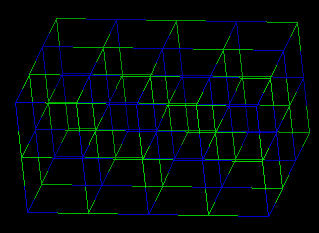 |
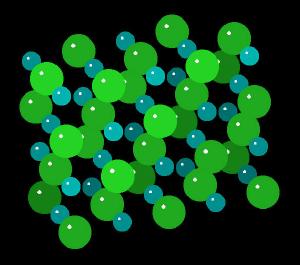 |
| Sodium Chloride: Wireframe | Sodium Chloride: Spacefilling |
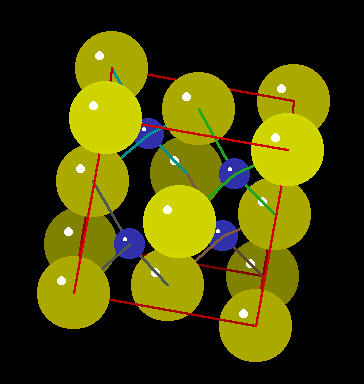
Zinc Sulfide
Zinc Sulphide (ZnS) is a mineral that emits light when struck by various types of radiation. Historically, it was used by Rutherford to detect alpha radiation bursts from radium and other radioactive minerals. It also can be incorporated into a variety of semi-conducting materials.
The zinc sulphide crystal Unit Cell (simplest spatial arrangement that is extended in all directions to form the crystal observed at the macro level) is an arrangement of covalently bonded zinc and sulfur.
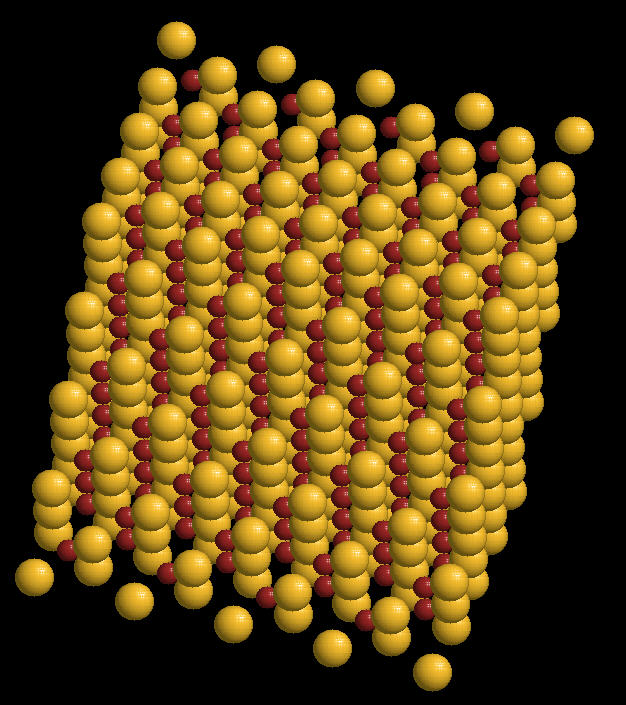
Zinc Sulphide Crystal: Spacefilling
Some Straight Chain Hydrocarbons
Hydrocarbons are compounds composed entirely of carbon and hydrogen atoms. Because carbon atoms can assume a variety of bonding configurations, hydrocarbons can assume a variety of shapes. The study of carbon compounds is a particular branch of chemistry called organic chemistry. The term organic is derived from the historical notion that such compounds could only be made by living things. We now know that this is incorrect.
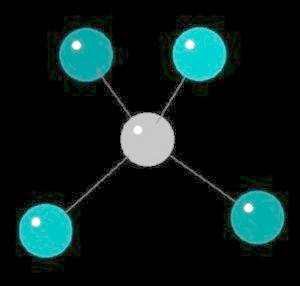
Methane (CH4), an odorless gas, is the primary component of natural gas. The strength of the covalent carbon hydrogen bond is among the strongest in all hydrocarbons. Methane, a biogas (derived from biological sources) is a greenhouse gas with a global warming potential 22 times the warming ability of carbon dioxide. It is estimated that each year 45,000,000 tons of methane are added to the atmosphere from the "venting" of intestinal gases of large animals. When methane is used as a fuel, small amounts of very strong smelling mercaptans (sulfur containing compounds) are added to allow detection of leaks.
Methane is the simplest alkane (Compounds with only single bonded carbon and hydrogen atoms).
The methane molecule is tetrahedral with a central carbon atom surrounded by 4 hydrogen atoms directed at the corners of a regular tetrahedron.
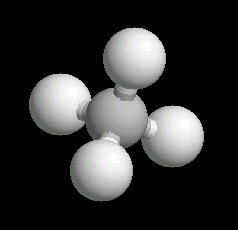 |
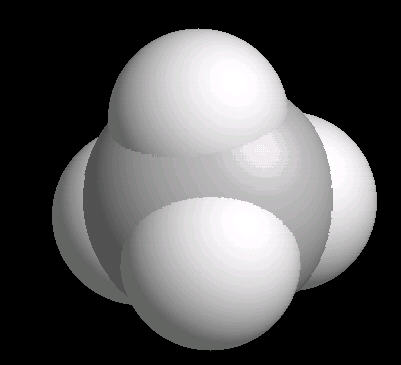 |
| Methane: Ball & Stick | Methane: Spacefilling |
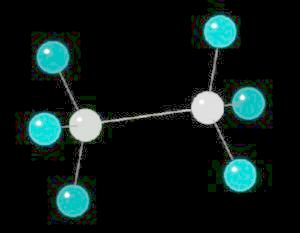
Ethane (C2H6) is the only 2 carbon alkane. It is an odorless gas; a component of natural gas. It's primary use is as a starting material for a variety of petrochemicals.
The ethane molecule can be considered as two tetrahedral carbon atoms joined together. There is free rotation around a C-C single bond, so the methyl groups (CH3) can be visualized as spinning around the axis of the C-C bond.
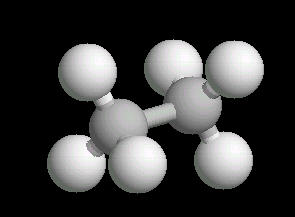 |
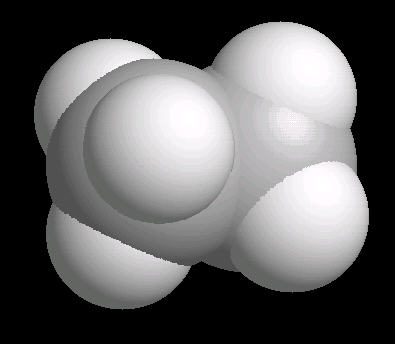 |
| Ethane: Ball & Stick | Ethane: Spacefilling |
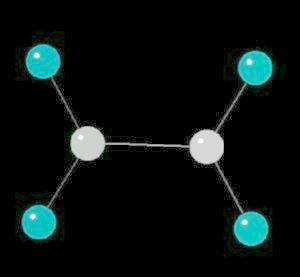
Ethylene (C2H4) is the simplest alkene (compounds containing only carbon and hydrogen with double bonded carbon atoms, C=C). Ethylene is a major starting material for a multitude of petrochemicals.
The C=C double bond is a rigid, planar arrangement.
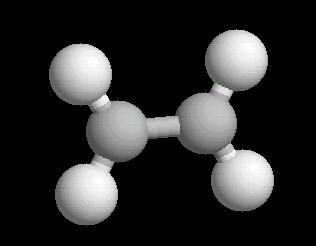 |
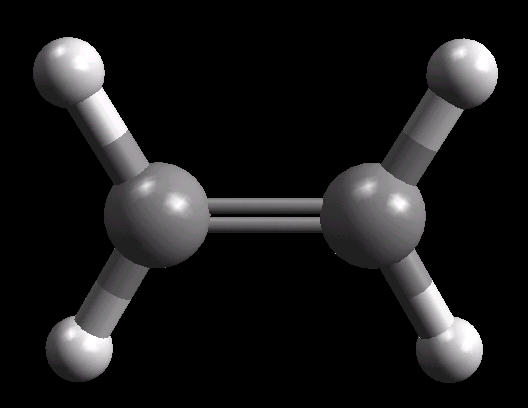 |
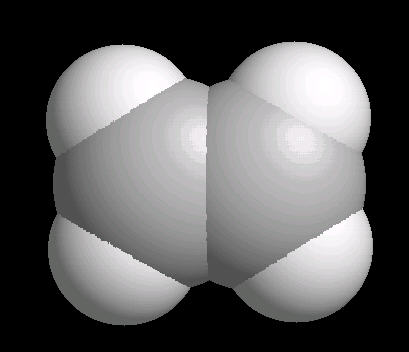 |
| Ethylene: Ball & Stick | Ethylene: Double Bonds | Ethylene: Spacefilling |
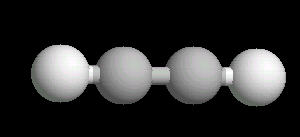
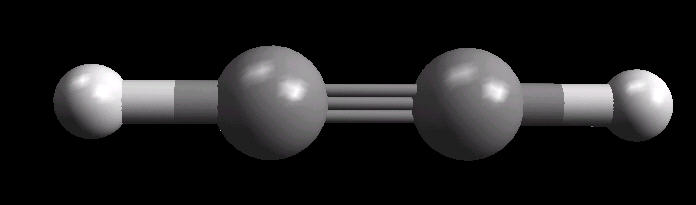
Acetylene.(C2H2) is the simplest alkyne (compounds containing only carbon and hydrogen with triple bonded carbon atoms, C=C). It burns with one of the hottest hydrocarbon combustion flames known. It also is an important industrial starting material for a variety of products.
The C=C triple bond is linear.
 |
 |
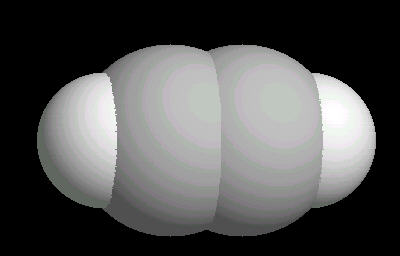 |
| Acetylene: Ball & Stick | Acetylene: Triple Bond | Acetylene: Spacefilling |
Propane.(C3H8) is a three carbon alkane. It is normally a gas, but is easily compressed to a liquid. It is a component of natural gas.
Its primary use is as a fuel.
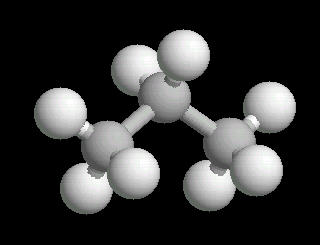 |
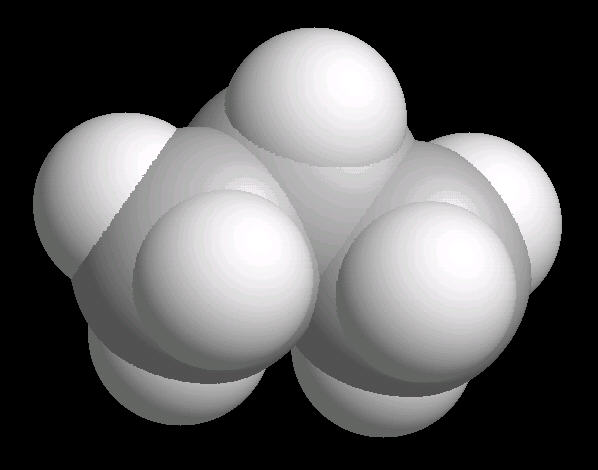 |
| Propane: Ball & Stick | Propane: Spacefilling |
Butane (C4H10) in the anti-conformation
Butane is the four carbon alkane.
Butane,
like all linear alkanes, is a colorless, odorless gas used for fuel.
The free rotation around the Carbon-2__Carbon-3 bond allows butane to assume several different spatial orientations or conformations. The Anti-conformation places the methyl (CH3) groups on Carbon-2__Carbon-3 the furthest apart. This is the most stable conformation of butane.
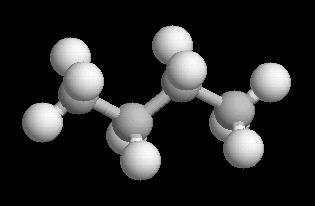 |
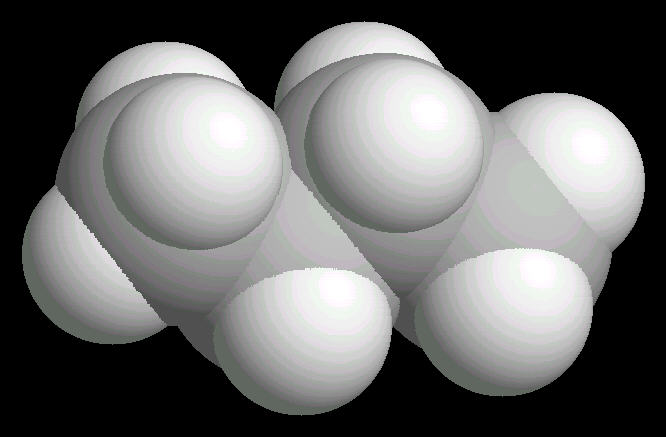 |
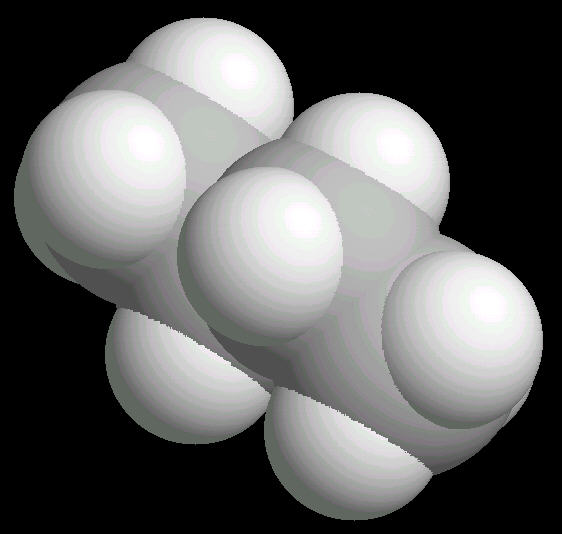 |
| Butane_Anti: Ball & Stick | Butane_Anti: Spacefilling | Butane_Anti: different view |
Butane (C2H10) in the eclipsed-conformation. If you rotate the anti-conformation around the axis of the Carbon-2__Carbon-3 bond, there is a conformation where the adjacent methyl groups become close to each other. This is called the eclipsed position. This position requires the most energy to maintain.
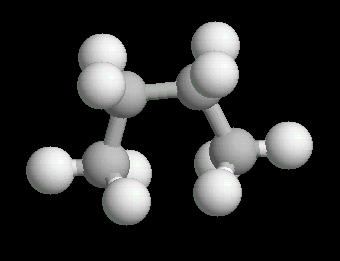 |
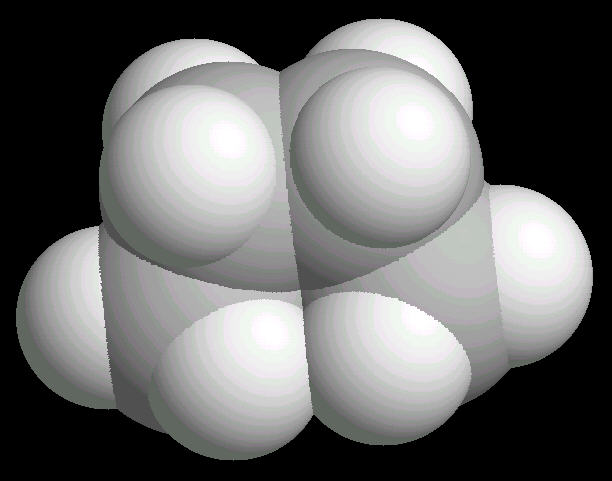 |
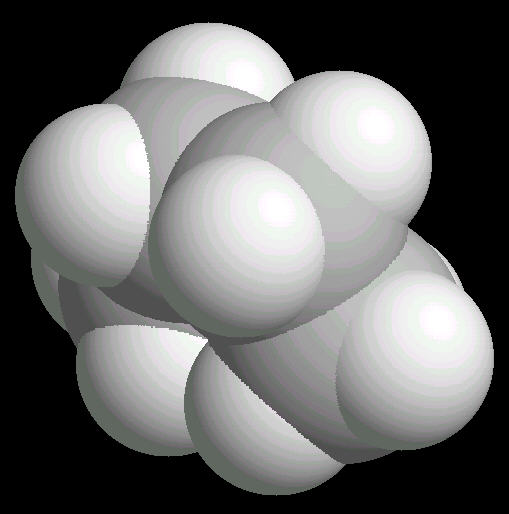 |
| Butane_Eclipsed: Ball & Stick | Butane_Eclipsed: Spacefilling | Butane_Eclipsed: different view |
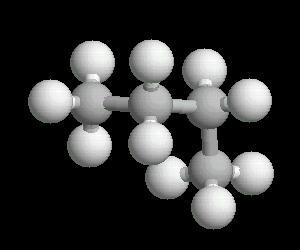
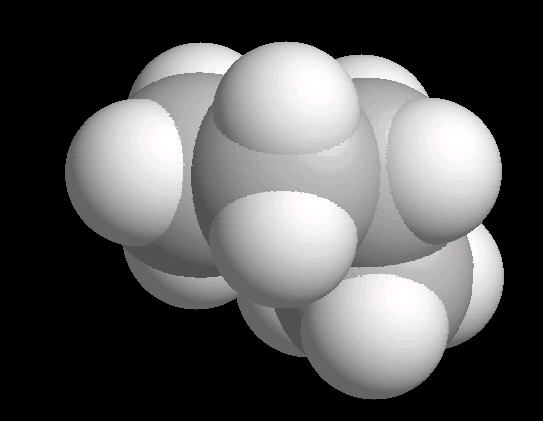
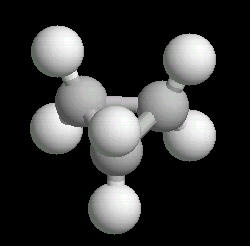
Butane (C2H10) in the gauche (staggered) conformation
In this arrangement (more energetic than anti, but less than eclipsed), the methyl groups assume a staggered arrangement.
 |
 |
 |
| Butane_Gauche: Ball & Stick | Butane_Gauche: Spacefilling | Butane_Gauche: different view |
Some Cyclic Hydrocarbons
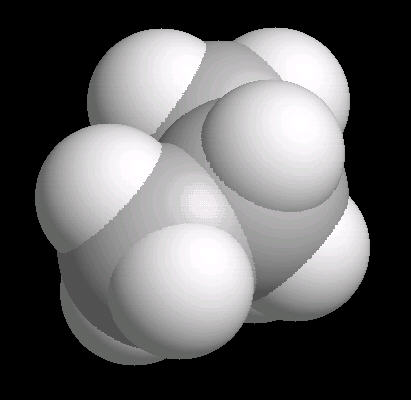
Cyclopropane (C3H6) is the
simplest cyclic alkane. The bonds in this cyclic structure are highly strained.
This provides an enormous amount of potential energy. As such, cyclopropane is a
very reactive molecule. Its primary use is as a synthetic starting material for
a wide variety of organic molecules.
 |
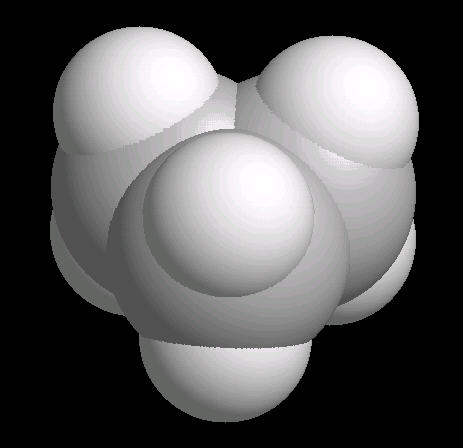 |
| Cyclopropane: Ball & Stick | Cyclopropane: Spacefilling |
Cyclobutane (C4H8) is
the cyclic four carbon alkane. As with cyclopropane, the carbon-carbon bonds are
strained.
The molecule, in an attempt to relieve some of the bonding strain, is not planar, but adopts a slightly "puckered" orientation.
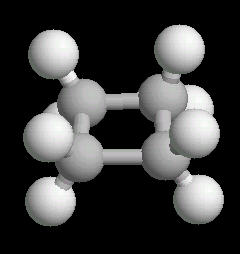 |
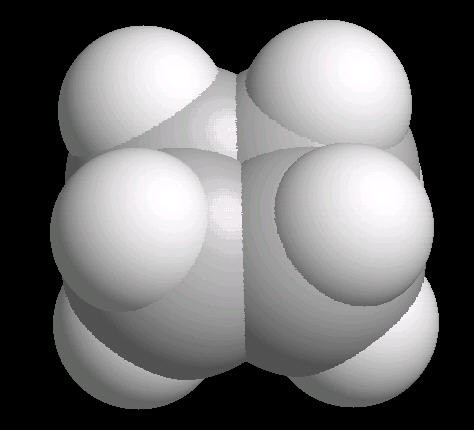 |
| Cyclobutane: Ball & Stick | Cyclobutane: Spacefilling |
Cyclopentane (C5H10) is the cyclic five carbon alkane. It is a highly flammable liquid which is used primarily as a starting material for various petrochemicals and resins. The carbon-carbon bonds are strained and this results in a "pucker" or non-planar orientation of the ring,
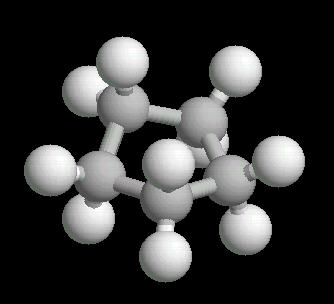 |
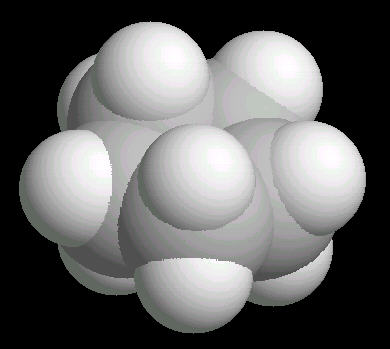 |
| Cyclopentane: Ball & Stick | Cyclopentane: Spacefilling |
Cyclohexane (C6H12) in the "boat" conformation
Cyclohexane is the cyclic six-member alkane. It is an important starting material for the synthesis of a large number of chemical compounds.
While the six member ring is in constant flexing motion, it has two notable conformations: boat and chair. The boat is shown below.
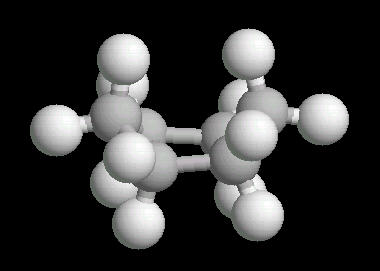 |
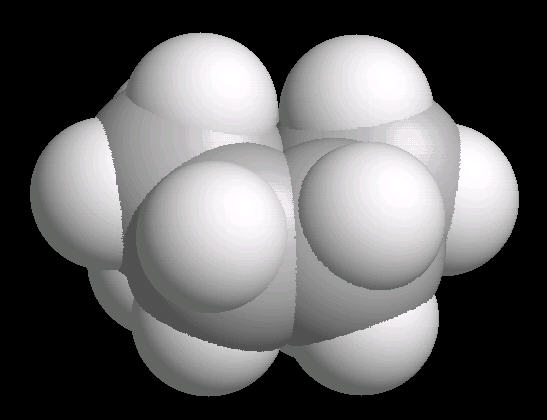 |
| Cyclohexane_Boat: Ball & Stick | Cyclohexane_Boat: Spacefilling |
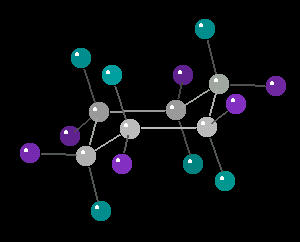
Cyclohexane (C6H12) in the "chair"
conformation
The chair conformation is the most stable form of a cyclic six member alkane ring. In this conformation, every atom is at its farthest possible point from all other atoms of the molecule. In this position, there are two types of hydrogen atoms: axial and equatorial. The differentiation between axial and equatorial is illustrated in the Cyclohexane_Chair kinemage.
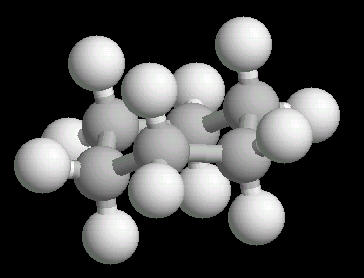 |
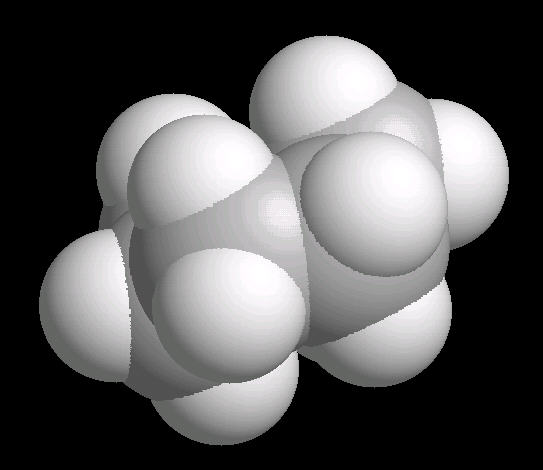 |
| Cyclohexane_Chair: Ball & Stick | Cyclohexane_Chair: Spacefilling |
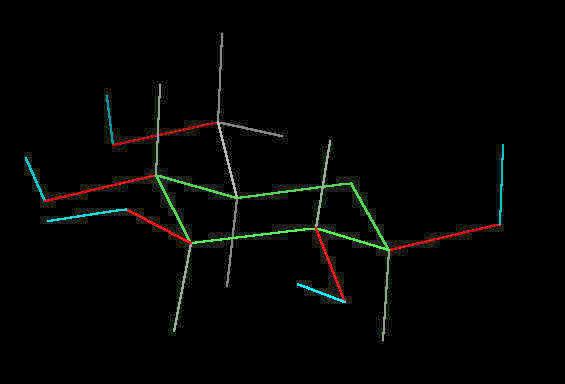
Glucose
Glucose (C6H12O6) is the primary sugar energy source. This molecule is a carbohydrate (composed of carbon, hydrogen and oxygen ... a "hydrated" carbon). The predominant form of this sugar is the "chair conformation. (The "chair" is quite common in many classes of naturally occurring substances).
The multiple surface-accessible polar OH (hydroxyl) groups explain the ease with which glucose dissolves in water..
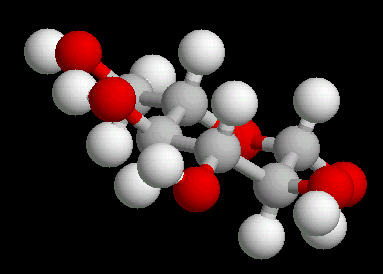 |
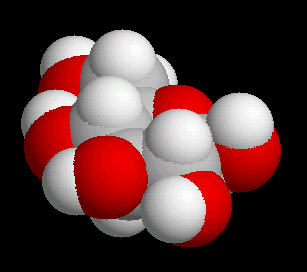 |
| Glucose_Chair: Ball & Stick | Glucose_Chair: Spacefilling |
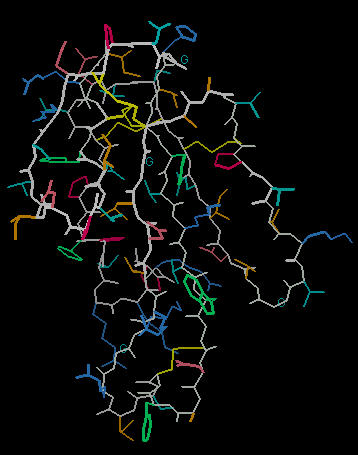
Explaining Biological Activity: Cobratoxin
The Cobra Toxin from the cobra snake ranks among the world's most lethal venoms. Unlike many protein-based neurotoxins, this venom is not denatured (disabled) by heat. The lethal nature of the venom and its heat stability can be explained by looking at the molecular model for this poison.
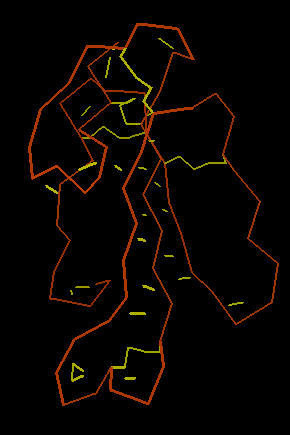
The image below left shows an outline of the amino acid backbone for the protein (orange). There are five disulphide bonds (yellow; S-S links between Cys residues) that hold the molecule into a relatively rigid structure. Five disulphide bonds within such a small protein is unusual, but common within the extremely toxic snake venoms. The image, below center, adds hydrogen bonds (short yellow lines) to the display . The combination of disulphide bonds and hydrogen bonds (particularly within the long center loop) holds the molecule into a shape that precisely fits into the post-synaptic nicotinic cholinergic receptor (one of the strongest protein binding interactions known). When the Cobra venom neurotoxin binds to the receptor, the receptor no longer functions. So, muscles that depend upon cholinergic signals fail to work; death by respiratory failure because the diaphragm cannot move the lungs.
The disulphide bonds are heat stable and will hold the molecule's shape under conditions that would denature (or change protein shape) most proteins.
The three-lobed structure (below right, shown in "ribbon" rendering that traces the shape of the molecule) has been called the "devil's trident" because this shape is present in many snake neurotoxins.
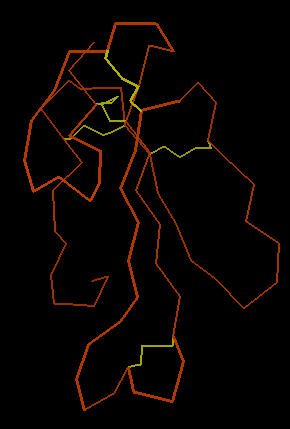 |
 |
 |
| Cobra Toxin: Disulfides | Cobra Toxin: Hydrogen Bonds | Cobra Toxin: Ribbon |
So, the cobra toxin is lethal because its rigid shape precisely fits into a vital neurotransmitter receptor site. Once bound, the neurotoxin acts as a plug to the receptor and prevents the bound receptor from initiating a neural impulse. The multiple disulphide bonds prevent the molecule from altering shape (to decrease its receptor binding efficiency) with heat.
Explaining Biological Activity: The FGF System
Finally, kinemages can be a useful research tool to explain chemical properties to others. Here is a my site using kinemages as a tool for understanding the fgf. system.
The Kinemages
The KiNG molecular model visualization software requires a java-enabled (JRE from Java) browser:
Possible Icons to the left of molecular model image below indicates the availability of the models to your browser:
| Java Not Activated | Java Not Activated | Java Functional |
 |
Blank Area
or message: Image requires a Java enabled browser
|
 |
| KiNG Inactive | KiNG Inactive | KiNG Full Functional |
For java enabled browsers, a single click on the KiNG logo will launch the appropriate kinemage. The number under the KiNG logo is the download size (in bytes).
Use the left mouse button to rotate the molecule. A single click on the KiNG logo will launch the appropriate kinemage.
If your browser has been Java-crippled, you can download the King application (KiNG DownloadIndex) to use on your local machine. All of the kinemages below which have been collected into a zipped archeive SimpleMolecules for use on a local machine (download the zipped arxhive, unzip, and open the selected kinimage file with the KiNG java applet). The Windows KiNG application used on this website is Here
|
1 K |
 |
| Click on KiNG to see | Ammonia |
|
1 K |
 |
| Click on KiNG to see | Carbon Dioxide |
|
1 K |
 |
| Click on KiNG to see | Carbon Monoxide |
|
24 K |
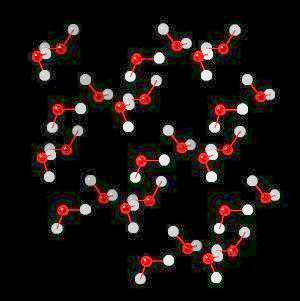 |
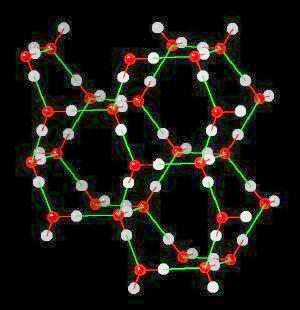 |
| Click on KiNG to see | Ice | Ice - with H-Bonds |
|
4 K |
 |
| Click on KiNG to see | Sodium Chloride |
|
3 K |
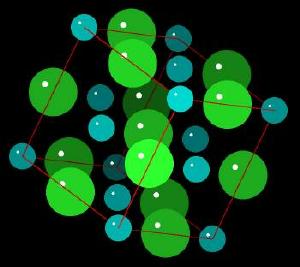 |
| Click on KiNG to see | Sodium Chloride |
|
36 K |
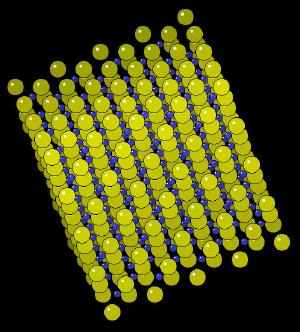 |
| Click on KiNG to see | Zinc Sulfide |
|
3 K |
 |
| Click on KiNG to see | Zinc Sulfide: Unit Cell |
Hydrocarbons
|
1 K |
 |
| Click on KiNG to see | Methane |
|
2 K |
 |
| Click on KiNG to see | Ethane |
|
2 K |
 |
| Click on KiNG to see | Ethylene |
|
1 K |
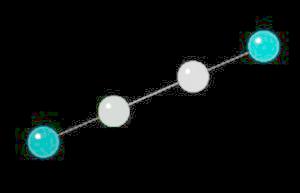 |
| Click on KiNG to see | Acetylene |
|
2 K |
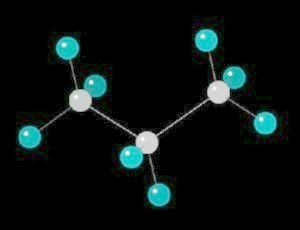 |
| Click on KiNG to see | Propane |
Butane in the Anti-Conformation
|
2 K |
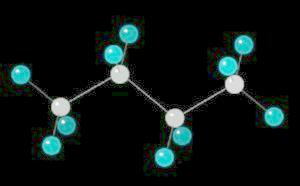 |
| Click on KiNG to see | Butane in the Anti-Conformation |
Butane in the Eclipsed-Conformation
|
2 K |
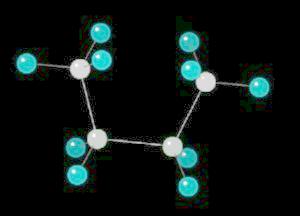 |
| Click on KiNG to see | Butane in the Eclipsed-Conformation |
Butane in the Gauche-Conformation
|
2 K |
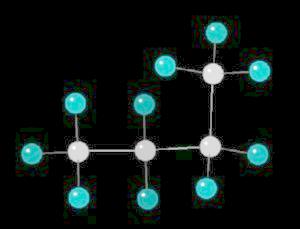 |
| Click on KiNG to see | Butane in the Gauche-Conformation |
|
2 K |
|
| Click on KiNG to see | Cyclopropane |
|
2 K |
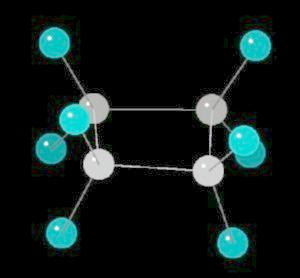 |
| Click on KiNG to see | Cyclobutane |
|
2 K |
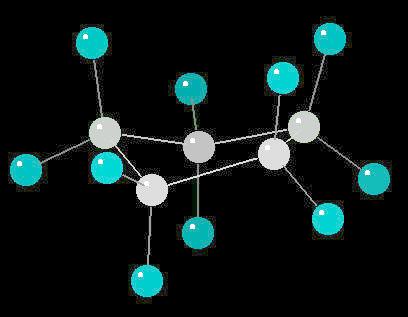 |
| Click on KiNG to see | Cyclopentane |
Cyclohexane in the Boat-Conformation
|
3 K |
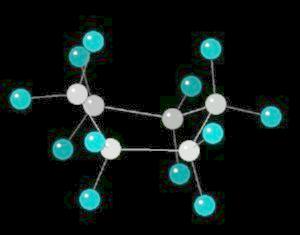 |
| Click on KiNG to see | Cyclohexane in the Boat-Conformation |
Cyclohexane in the Chair-Conformation
|
3 K |
 |
| Click on KiNG to see |
Cyclohexane in the Chair-Conformation |
|
4 K |
 |
| Click on KiNG to see |
Glucose in the Chair-Conformation |
|
121 K |
 |
| Click on KiNG to see |
Cobra Toxin |
My
UniversityHome
Harris
Links Chemistry
/ Modeling Links
Copyright 2020 by Larry P. Taylor
All Rights Reserved