| Home |
| Basic Theory and Weak Statement |
| Computational Design |
| Matlab Methods |
| Results |
| Basic Theory and Weak Statement |
Overview of Theory Starting with a solid phase particle that is in contact with vapor, atoms will either condense onto the vapor or atoms from the particle will evaporate into the vapor causing the particle to either grow or shrink, respectivly moving the interface. Whether or not a particle grows or shrinks depends on the free energy of the system, the way the inferface behaves is determined by the minimization of the free energy of the system. Free energy is given by the equation:
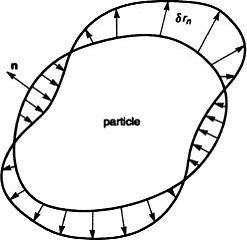
Above is a figure of an example of such a particle. The interface moves in the direction of n, which is the normal direction with respect to the particle surface. The amount of motion is denoted by δrn. This motion can differ point to point on the surface (just as the figure above) which gives raise to the variation of the free energy which is denoted as δG, given by the equation: P is defined as a thermodynamic force (driving pressure) and dA is the differential area. The above equation defines the quantity P at every point. The next step is kinetic law, where νn is the velocity in the direction normal to the interface. The velocity is taken as a linear function of the driving pressure. To validate the linear relationship it is assumed that ΩP<<kT, where Ω is the atomic volume, k is the Boltzmann constant, and T is the absolute temperature. The velocity is given by, where L is a kinetic coefficient:
Now by using the weak statement the equation above can be plugged into the δG equation to give:
When taking surface tension, γ, into account νn becomes:
Here K is the curvature of the particle and g is the specific energy between the two phases (would be zero if no phase change). Now by plugging in the new νn into the new δG equation yields:
|