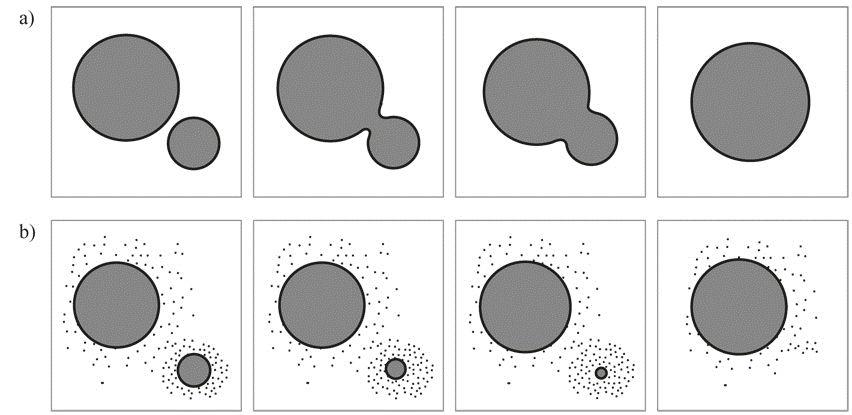
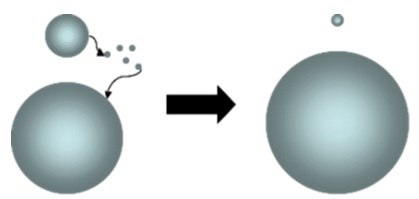
Coarsening process, also called Ostwald ripening, is an observed phenomenon in solid solutions or liquids which describe the change of an inhomogeneous structure over time, i.e., small crystals or sol particles dissolve, and redeposit onto larger crystals or sol particles. [1]
Dissolution of small crystals or sol particles and the redeposition of the dissolved species on the surfaces of larger crystals or sol particles were first described by Wilhelm Ostwald in 1896.[2][3]
This thermodynamically-driven spontaneous process occurs because larger particles are more energetically favored than smaller particles.[4] This stems from the fact that molecules on the surface of a particle are energetically less stable than the ones in the interior. Large particles, with their lower surface to volume ratio, results in a lower energy state (and have a lower surface energy). As the system tries to lower its overall energy, molecules on the surface of a small (energetically unfavorable) particle will tend to detach and diffuse through solution and then attach to the surface of larger particle. Therefore, the number of smaller particles continues to shrink, while larger particles continue to grow.
An everyday example of Ostwald ripening is the re-crystallization of water within ice cream which gives old ice cream a gritty, crunchy texture. Larger ice crystals grow at the expense of smaller ones within the ice cream, creating a coarser texture.

Friedrich Wilhelm Ostwald (2 September 1853 – 4 April 1932) won Nobel Prize for Chemistry (1909)
In chemistry, Ostwald ripening refers to the growth of larger crystals from those of smaller size which have a higher solubility than the larger ones. In the process, many small crystals formed initially slowly disappear, except for a few that grow larger, at the expense of the small crystals. The smaller crystals act as fuel for the growth of bigger crystals. Limiting Ostwald ripening is fundamental in modern technology for the solution synthesis of quantum dots (the nanoparticles).
Ostwaid ripening (If you are using IE explorer, to watch the movie, please click "allow blocked contents" on the top of your windows)
Simulation of a system full of particles of different sizes is too complex to start with. We decided to simplify the problem and see what we can do with what we learned in the course. We begin with one of the cases, where there are only two particles, growing independently and merging into a bigger particle. Assuming the particle growth is driven by diffusion and interface migration, we want to look at by tracking the interface, whether a 2D concurrent simulation could capture the behavior of the system.
© Copyright 2013 All rights reserved. No part of this publication may be reproduced or transmitted without the express written consent of the authors.

