
Nerve Tissue Co-culture
Robert G. Dennis, Ph.D.
 |
Nerve Tissue Co-culture Robert G. Dennis, Ph.D. |
 |
My research in nerve tissue engineering involves four general areas:
1- Nerve-Muscle co-culture to promote synaptogenesis
in engineered skeletal muscle.
[Collaboration with
Marlene Calderon, M.D.]
2- Gene expression and synaptogenesis.
[Collaboration with
Dan Goldman, Ph.D., and Peter Macpherson, Ph.D.]
3- Monitoring and depolarization of cultured
nerve axons using sieve electrodes.
[Collaboration with
Dave Anderson, Ph.D. and Jamie Hetke of the Center
for Neural Communication Technology]
4- Acellularized nerve tissue ECM for use
as a nerve conduit.
[Collaboration with
Bill Kuzon, M.D., Ph.D., and Paul Cederna, M.D.]
These four areas of research in nerve tissue engineering all relate to different aspects of the technology development and ultimate clinical application of engineered functional skeletal muscle tissue.
In a collaboration with Marlene Calderon, M.D. we were able to develop a nerve-muscle co-culture system in which axonal projections from a ventral horn explant projected to the muscle construct in vitro, as shown in the image below.
Purpose: to promote enhanced
excitability and contractility of the engineered muscle constructs by providing
nerve-derived trophic factors and synaptic stimulation at the neuromuscular
junction.
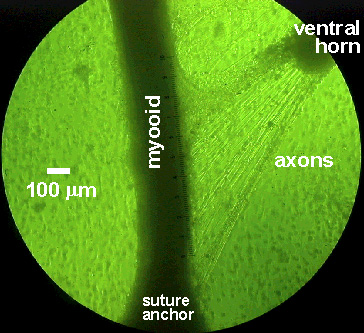 |
Nerve-muscle co-culture system.
We were able to get axonal projections from the ventral horn spinal explants
from embryonic rats to project to the myooid muscle constructs in culture.
This photo is representative of many successful nerve-muscle co-culture
systems. When tested for changes in contractility or tissue excitability,
there were no differences between muscles in co-culture and muscles cultured
entirely aneurally. We also were not able to detect synaptogenesis
by cholinesterase staining at the putative motor end plates. We therefore
hypothesize that the muscle constructs are not receptive to synaptogenesis.
Our working hypothesis is that the muscle construct must be pre-conditioned
by electrical stimulation to promote the correct expression of nicotinic
acetylcholine receptors for the muscle tissue to be receptive to reinnervation
(see Dan Goldman collaboration below).
The scale running parallel to the myooid muscle construct is 1 mm long, with 100 divisions. The myooid is approximately 300 microns in diameter. Photo by Marlene Calderon. |
In a collaboration with Dan Goldman and Peter Macpherson, our objective is to control the relative expression of different subunits (g and e) of the nicotinic acetylcholine receptor (nAChR) expressed by the muscle, to allow re-innervation of the skeletal muscle after long periods of denervation. Prolonged denervation of skeletal muscle is typified by a reversion to a state where the muscle expresses subunits that are not receptive to synaptogenesis. Peter has used the implantable stimulator system that I have designed and has demonstrated that, by controlled intermittent muscle cell membrane depolarization, he can control and reverse the relative levels of expression. He is currently executing experiments to demonstrate an increased potential for reinnervation of the long-term denervated skeletal muscles in vivo (rats). Marlene Calderon is also working on a doctoral project which uses my implantable stimulators to maintain muscle mass and contractility during long-term denervation. For my part in this collaboration, in addition to designing and providing the electrical stimulators, I will test this hypothesis in vitro in a nerve-muscle co-culture system. The current engineered muscle model is cultured entirely aneurally (no innervation), and therefore may be refractory to innervation unless properly preconditioned by depolarization activity.
Objectives: (1) Permit depolarization of individual motor axons in culture to achieve motor unit level control of engineered muscle tissue, (2) exert control over the phenotype of muscle fibers in each motor unit, and (3) monitor spontaneous depolarization of individual motor axons during development in vitro.
Experimental Plan: Sieve electrodes
coated with laminin and other surface adhesion molecules will be interposed
between the nerve tissue and the muscle construct in culture. The
iridium metal electrode surfaces form an oxide layer, and allow a pseudo-capacitive
electrode coupling to the tissue. Due to the many iridium oxide transitions,
the electrodes can provide an adequate stimulus to discharge a motor axon.
The sieve electrodes can be designed to accommodate any desired geometry,
with an arbitrary number of instrumented holes, each hole of arbitrary
dimensions. A representative photograph of a currently available
sieve electrode is shown in the photograph below. Nerve tissue source
will be either primary ventral horn explants (as shown above) or PC12 cells,
which are known to form synapses with muscle cells in culture.
| The image to the right is of a sieve electrode specifically designed for in vivo implantation at the site of a nerve transsection. We are currently collaborating to develop a sieve electrode specifically for the nerve-muscle co-culture configuration shown above. The probes are fabricated by the University of Michigan Center for Neural Communication Technology, sponsored by NIH NCRR grant P41 RR09754, for Dr. Steven Highstein of Washington University. (see: Mensinger, et al., J. Neurophys 83: 611-615, 2000). |  |
In a collaboration with
Bill Kuzon and Paul Cederna, I have developed a protocol for complete acellularization
of tissues with tubular cell components, such as muscle and nerve.
The purpose for the acellularization process is to provide a scaffold of
extracellular matrix proteins for cell ingrowth to guide nerve axon regeneration
over long gaps (> 2 cm). The candidate tissues for the neural conduits
are muscle and nerve. The acellularization process that I have developed
works equally well with both tissue types, entirely removing all cellular
components while leaving the ECM intact. A representative photograph
showing the effectiveness of this procedure is shown below, using a full
section thickness of mouse EDL muscle, which is approximately 3 mm thick.
Before the acellularization process, the tissue is opaque, after the process
it is transparent because only the ECM remains after all cellular components
have been removed. When segments of nerve tissue are subjected to
the same process, an acellularized structure results that has the correct
architecture to support Schwann cell repopulation and axonal regeneration.
In preliminary studies we have found that the acellularized nerve conduits
are entirely non-immunogenic when transplanted between animals with major
histocompatibility mismatches. The acellularized tissue elicits a
less severely immune response than autograft tissue (due probably to the
presence of necrosing cells in the autograft, even though there is no rejection
of xenogenetic material in the autograft tissue, per se.) Our preliminary
data also indicate that the acellularized nerve conduits will support Schwann
cell repopulation and axonal regeneration.
 |
 |
|
|
|
Significance: The ability to create nonimmunogenic acellular nerve conduits that support axonal regeneration over large gaps has direct clinical significance because it then becomes possible to use cadaveric nerve tissue (in plentiful supply) to produce the graft material for a patient, which can then be repopulated by the patient's own Schwann cells prior to reimplantation, so axonal regeneration will be supported and there will be no tissue rejection.
Bob's Home Page Current Research Muscle Mechanics Lab (U of M) Biomechatronics Group @ MIT