
Muscle Tissue Engineering
Robert G. Dennis, Ph.D.

 |
Muscle Tissue Engineering Robert G. Dennis, Ph.D. |
 |
Objective: To engineer functional contractile mammalian skeletal muscle in vitro.
Experimental Approach:
My principle collaborators
in this effort are Paul Kosnik, Ph.D. and Herman Vandenburgh, Ph.D.
Herman has a lengthy history of functional skeletal muscle tissue engineering,
his first papers in the area being published in the 1980's. My collaboration
with Hugh Herr is focused on robotic actuator technologies involving engineered
muscle, and includes muscle tissue from many sources other than mammalian.
The experimental approach
developed by Paul and I centered on developing self organizing muscle
tissues, that is, employing no artificial scaffolds for the contractile
segment of the engineered muscle. In 1997 and 1998 we developed a
baseline model for engineered muscle in vitro that was self organizing,
and generated easily measurable force. We term these engineered skeletal
muscle constructs myooids, because they are muscle like in
form and function. The self organization of myooids in culture is
shown below. Note that the muscle remains attached at each end to
a silk suture anchor. The suture anchors serve as artificial tendons,
allowing the muscle to be manipulated and attached to force transducers
and servo motors without injuring the muscle.
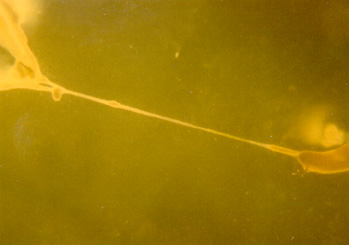 |
The image to the left is a photograph
of the first myooid ever produced in our laboratory (July 7, 1997).
The myooid is attached at each end to acellularized skeletal muscle anchors,
pinned in place by 0.10" diameter insect pins. This myooid is approximately
30 mm in length, from anchor to anchor. It was cultured in a 100
mm diameter culture dish, with an uncoated Sylgard substrate.
The myooid was seen to spontaneously contract immediately after each feeding. No functional data were measured, as the instrumentation was not in place at that time. (Photograph by R.G. Dennis and P. Kosnik, 1997) |
 |
 |
 |
 |
| 1. Monolayer of muscle cells detaching from the substrate and rolling into a cylinder. This process requires several days. Dish diameter is 35 mm. | 2. Approximately 3 days after delamination of the monolayer, the cells have self organized into a cylinder. | 3. Representative cross section of a myooid, stained with 1% Toluidine blue. The scale bar is 100 mm. Note the annulus of fibroblasts surrounding the muscle cells. | 4. Representative cross section of a myooid, stained with 1% Toluidine blue. The scale bar is 100 mm. |
 |
 |
 |
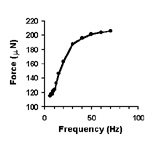 |
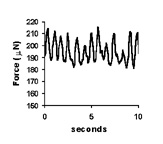
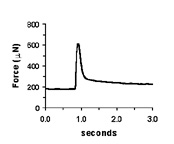
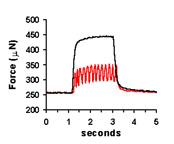
| Spontaneous contractions of a myooid, ~1 Hz. | A single twitch, elicited by a single, 70V, 0.4 ms pulse. | Tetanic contractions at 30 Hz (black trace) and 6 Hz (red trace). | Force frequency diagram. As stimulation frequency is increased, tetanic force increases. |
 |
 |
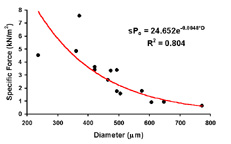 |
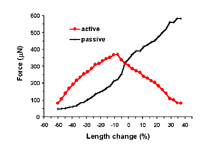
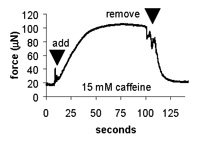
| Length-tension curve. Note that the passive curve (black) is left shifted from control values. The peak of the active curve (red) occurs at -5% to -10% of the cultured length. | Effect of caffeine addition on the contractile force of myooids. this demonstrates that myooids could be used in a contractility assay to diagnose malignant hyperthermia. | Diffusion limits the size of avascular engineered skeletal muscle. The maximum diameter is generally 500 mm. Myooids with diameters greater than this have necrotic cores, with essentially non-contractile muscle. |
 |
 |
Internal forces generated within a
myooid:
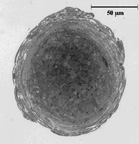
Myooids are a co-culture of at least two cell types; myoblasts (which form long tubes) and fibroblasts. During self organization, the fibroblasts are essential for forming the extracellular matrix material that holds the structure together. After formation, fibroblasts tend to organize around the periphery of the myooids, as shown to the left. Based on our passive force data, and using a multiple regression model (assuming biaxial tension generation by the fibroblasts), we calculate that the fibroblasts exert a continuous 5 kPa stress, which acts both longitudinally and as a hoop stress. The hoop stress results in an internal pressure of ~ 1 kPa on the myotubes at the core of the myooid. |
| Myooid from adult muscle,
~ 7% fibroblasts in the cross section. |
Myooid from neonatal
cells, ~ 30% fibroblasts in the cross section. |
 |
 |
 |
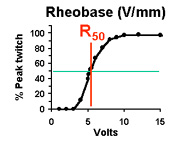
| Bulk excitability of an engineered muscle
Rheobase (R50): Stimulus amplitude required to elicit a 50% peak twitch force. (measured at long stimulus pulse duration) |
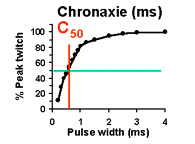
Bulk excitability of an engineered muscle
Chronaxie (C50): Stimulus duration required to elicit a 50% peak twitch force. (measured at 2x R50) |
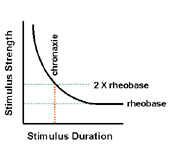
Classical definition of excitability for nerve and muscle tissues. The combination of stimulus duration (chronaxie) and stimulus strength (rheobase) must lie above the curve to elicit depolarization of the cell membrane. |
Based on our measurements of the excitability of engineered tissues, the values for R50 and C50 are each approximately 1 order of magnitude greater than control muscle tissues. High values of C50 and R50 indicate LOW tissue excitability, which is undesirable. This is understandable, given that the engineered tissues are cultured entirely in the absence of innervation. The consequence of this very low excitability is that the energy required to stimulate the tissues is much greater than required for normally innervated muscle; it is in fact ~1000 times greater, calculating the energy requirements directly from Ohm's law. Thus, it is critical to improve the excitability of engineered skeletal muscle tissue if the tissue is ever to be exposed to chronic electrical stimulation in culture, or if it is to function as a robotic or prosthetic actuator.
NOTE: (if
the movie opens in Netscape navigator)
To play the following
movie, just left click on the image after it loads.
To return, use
the BACK button on your browser.
To pause or move
frame-by-frame during viewing, right click on image.
Discussion of Movie: Note
that in this case the myooid has naturally reduced the stress concentration
at its attachment point by increasing its section thickness gradually as
it inserts into the suture. The muscle fibers interdigitate into
the silk fibers of the suture. The spontaneous contractions are vigorous
and occur at a high frequency (several Hz) because the culture media surrounding
this myooid has just been replaced. In a short while, the spontaneous
contractions will tend to slow to ~ 1 Hz.
Bob's Home Page Current Research Muscle Mechanics Lab (U of M) Biomechatronics Group @ MIT