 |
Diving
Myths Dive Physics
Solutions To Problem Set 2 Larry "Harris" Taylor. Ph.D. Diving Safety Coordinator University of Michigan Ann Arbor, Michigan
|
 |
 |
Diving
Myths Dive Physics
Solutions To Problem Set 2 Larry "Harris" Taylor. Ph.D. Diving Safety Coordinator University of Michigan Ann Arbor, Michigan
|
 |
Go To: Home About "Harris" Articles Slides War Stories Editorials Links Fini
Different fonts may display the equations with inappropriate alignment. This page was assembled using the Arial 10 point.
1. Rather than memorize hundreds of conversions, it is best to memorize only a few and make all conversions as a long string of conversions to change from given to final:
a. 100 m x 60 sec x 100 cm x 1 in x 1 ft x 1 mi = 23.3 mi/hr
9.6 sec min m 2.54 cm 12 in 5,280 ft
b. 1 mi x 60 min = 15 mi/hr
4 min hr
c. 400 yd x 60 min x 3 ft x 1 mi = 1.1 mi/hr
12 min hr yd 5280 ft
2. 100 ft x 12 in x 2.54 cm x 1 m = 0.33 m/sec
92 sec 1 ft 1 in 100 cm
100 ft x 60 sec x 60 min x 1 mi = 0.74 mi/hr
92 sec min hr 5,280 ft
Measuring time to travel a short distance is a common method for determining current velocity.
3. A camera focus is set at the apparent distance (actual distance altered by the refraction of light). The refraction of light underwater results in the water acting as a lens that makes everything appear 75 % closer. So, the focus is set at:
0.75 x 6 ft = 4.5 ft
4. First, convert units to area to make the comparison
Jox = 30 ft x 60 ft = 1800 ft2
Field = 132 ft x 300 ft = 34,600 ft2
Next, determine ratio ... looking for Jox per football field
Jox x 34,600 ft2 = 22 Jox / field
1800 ft2 field
This is a bad pun. In the United States, the slang term for an athlete is "jock," which, in English, sounds like "jox." In addition, a US football team is 11 players, so a game of American football has 2 teams of 11 players each, or 22 jocks on the field.
5. First, determine the funds needed
5 ibs x 12 oz x $35.00 x 8 = $16,800.00
lbs oz
Next, determine amount of time need to earn this amount of money
$16,800.00 x 100 cents x hr = 112,000 hrs
dollar 15 cents
Convert to years
112,00 hrs x 1 day x 1 year = 12.79 years
24 hrs 365 days
6. Dew point is the temperature at which the saturated water vapor in the air must condense. Dew point is commonly reported in weather reports as an indicator of the temperature at which fog will form. Since the 52 Fo is below the reported dew point of 56 Fo, the warm, saturated air from your breath will be visible as soon as it cools to below dew point.
7. a. The temperature difference between surface and altitude:
70o F - 50o F = 20o F
So, the temperature difference corresponds to:
20o F x 1000 ft = 4,500 ft
4.5o F
b. For 2 degree drop every hour, the time involved for a 10 degree 68o F - 58o F) drop:
1 hr x 10o F = 5 hr
2o F
Five hours from 9 pm:
9 + 5 = 14 ==> 2 am on the 12 hour clock; 02 on the 24 hour clock
Since 2 am is later than midnight, the eclipse should be visible.
8. 100 g x 1.0 cal x 10o C = 1000 cal (or 1 kcal)
g oC
9. The meal calories total to
605 kcal + 214 kcal + 332 kcal = 1151 kcal
So, the time needed to "burn" 1151 kcals:
1151 kcal x 1 min = 127.9 min
9 kcal
10. The temperature difference needed to overcome the hypothermia state and restore normal body temperature:
98.6 oF - 90.0 oF = 8.6 oF
The heat content this represents:
60 kcal x 8.6 oF = 516 kcal
oF
The amount of water at 110 oF that holds this amount of heat:
516 kcal x 1 liter = 30.4 liters
17 kcal
Drinking approximately 30 liters to raise the temperature suggests that drinking warm water is not a viable hypothermia treatment.
11. a. Heat capacity (Cp) is a measure of the amount of heat a particular substance can absorb at constant pressure. The less the heat capacity, the less the tendency to absorb heat from the surroundings and the better the substance will serve as an insulator Assuming 100 g of gas is raised 10 degree Celsius.
For argon:
100 g x 0.1252 cal x 10 oC = 125.2 cal
g oC
For Air:
100 g x 0.3439 cal x 10 oC = 243.9 cal
g oC
For Helium
100 g x 4.9680 cal x 10 oC = 4,968 cal
g oC
b. Carbon dioxide reacts with water from perspiration to form a skin irritant, carbonic acid. The skin irritation from the formed acid suggests that using carbon dioxide as heat insulator inside a dry suit is not a particularly pleasant technique. The chemical reaction involved is
CO2 + H2O = H2CO3
12. Density = Mass / Volume
Since the mass of a gas stays constant and the volume (Boyle's Law) decreases with depth, the density must increase.
Using Boyle's law with density (D) substituted for Volume:
P1D1 = P2D2
Gas laws require absolute pressure:
Pabs = Pambient + P atmosphere
For the surface:
Pabs = 0 + 1 ata = 1 ata
At depth (1 atm corresponds to a pressure equivalent of 33 feet of sea water):
Pabs = ( 78 fsw x 1 atm ) + 1 atm = 2.36 atm + 1 atm = 3.36 ata
33 fsw
So, the Boyle's Law expression becomes:
(1 ata) (Dsurface) = (3.36 ata)(Dat depth)
Thus, gas is 3.36 times as dense at 78 fsw as it is on the surface.
Gas density contributes to breathing resistance and the work associated with breathing. This is a good reason to dive with top-of-the line regulators.
13. The height of a column of a substance is inversely proportional to its density.
(Remembering that 1 atm is defined as 760 mm Hg)
For an air barometer:
0.0012 g/cm = 760 mm
13,6 g/cm height for air
height of air = 8.61 x 106 mm
Converting units
8.61 x 106 mm x 1 m x 100 cm x 1 in x 1 ft x 1 mi = 5.35 miles
1000 mm 1 m 2.54 cm 12 in 5,280 ft
So, most of the weight of the earth's atmosphere is in the volume between the surface and 5.35 miles above the surface.
14. Depth gauges measure pressure, so depth readings must first be converted to a pressure.
For
seawater: 1 atm = 33 fsw
fresh water: 1 atm = 34 ffw
So, ambient pressure of 100 fsw:
100 fsw x 1 atm = 3.03 atm
33 fsw
Thus, the fresh water gauge will read a water pressure equivalent to 3.03 ata
3.03 atm x 34 ffw = 103 ffw
1 atm
Fresh water has no dissolved salts, so it will take a greater depth to reach an equivalent salt water pressure. Since diver depth gauges can be calibrated in either ffw or fsw, it is wise to check your gauges and dive the depth gauge calibrated to your diving environment.
15. Although
there are faster ways to solve this problem (e.g. memorizing a specific
formula), I prefer to take the solution in several logical steps so that,
regardless of a particular situation, understanding of what is known,
what is unknown and how I convert
the units of known to the unknown will direct my solution.
Depth
of a 1 atmosphere column of water will be inversely proportional to specific
gravity:
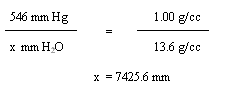
Converting to feet fresh water
![]()
The water pressure at 93 feet of fresh water
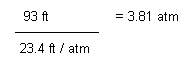
This corresponds as an absolute pressure of:
3.81
atm + 1 atm = 4.81 atm total pressure (ata) at altitude
The
pressure in mm Hg at this altitude would be:
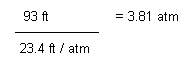
This hydrostatic pressure at sea level (where
1 atm is 760 mm Hg) would be:
Converting to a sea level pressure:
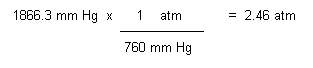
Converting
sea level atm to salt water depth:
2.46
atm x 33 fsw / atm = 81.2 fsw
3.82
atm x
33 fsw / atm = 126.1 fsw
The 126.1 depth is rounded to the next greater 10 foot entry, so as divemaster, you recommend that Indy's dive be controlled by the 130 fsw entry allowing him 10 minutes to find and recover the headpiece.
Thus,
Indy will have to dive to 93 feet of fresh water at an altitude near 9000 feet
above sea level, his depth gauge will read 81 fsw and he will treat the dive as
if his decompression schedule obligation was determined by a sea level dive of
130 fsw.
16. a. On Earth
67 fsw x 1 atm = 2.03 atm ===> 3.03 ata
33 fsw
On Xandor
Determine equivalent water depth to equal 1 atm (680 mm Hg compared to 760 mm Hg on Earth)
680 mm Hg = 1.47 g/cc
x mm H2O 13.47 g/cc
x = 6,231 mm
Converting to feet
6,231 mm x 1 cm x 1 in x 1 foot = 20.44 fsw
10 mm 2.54 cm 12 in
Determine absolute pressure of Xandor at 67 feet
67 fsw x 1 atm = 3.27 atm ===> 2.27 ata
20.44 fsw
b. Depth gauges read pressure
In terms of mm Hg for Xandor
3.27 ata x 680 mm Hg = 2,223.6 mmHg
ata
Converting to Earth Equivalent for absolute pressure
2,223.6 mm Hg x 1 ata = 2.92 ata (which is 1.92 atm of water depth)
760 mm Hg
So, earth gauge reading will be
1.92 atm x 33 fsw/atm = 63.5 ==> 64 fsw
c. Round to next highest 10 foot increment = 70 fsw schedule
d. on the bottom at 2700 psig and wishing to return at 1000 psig leaves 1700 psig for the dive.
1700 psig x ata-min x = 14.97 min ===> 15 minutes
50 psig 2.27 ata
e. The force diagram for earth
weight = 225 lbs ![]()
buoyancy = ? ![]()
Net force = 12 lbs ![]()
so, buoyant force on earth was 225 lbs - 12 lbs = 213 lbs ![]()
This corresponds to a volume of sea water at
213 lbs x 1 ft3 = 3.33 ft3
64 lbs
Determine buoyant force associated immersed Xandor volume of 3.33ft3
3.33 ft3 x (12 in)3 x (2.54 cm)3 x 1.47 g 1 lbs = 305 lbs
ft3 1 in3 cc 454 g
Set up forces diagram for Xandor
weight = 225 lbs ![]()
buoyancy = 305 lbs ![]()
Net force = 70 lbs ![]()
So, to dive in Xandor, the diver would need 70 lbs of lead to compensate for the buoyancy associated with diving in a dense sea
17. Depth (in ata) = Pressure (ata) / Fraction oxygen
For 1.6 ata
On Oxygen
D = 1.6 ata / 1
D = 1.6 ata
D (in water) = 0 .6 x 33 fsw/atm = 19.8 fsw
On Air
D = (1.6 ata) / 0.21
D = 7.62 ata
D (in water) = 6.62 atm x 33 fsw/atm = 218 fsw
For 1.4 ata
On Oxygen
D = (1.4 ata) / 1
D = 0.4 ata
D (in water) = 0.4 atm x 33 fsw/atm = 13.2 fsw
On Air
D = (1.4 ata) / 0.21
D = 6.67 ata
D (in water) = 5.67 atm x 33 fsw/atm = 187 fsw
18. Need both absolute pressure and temperature for gas law related problems
Absolute Temps:
45 oF = 45 oF + 460 oF = 505 oR
78 oF = 78 oF + 460 oF = 538 oR
Absolute Pressure = gauge plus atmospheric; in psi:
Pabsolute = 3000 psig + 14.7 psi = 3014 psi
Using law of Charles for determining pressure-volume at constant temp
P1 / T1 = P2 / T2
Substituting
3014 psi = P2
505 oR 538 oR
P2 = 3210.95 psi (This is absolute pressure)
Converting to gauge pressure:
3210.95 psi - 14.7 psi = 3196.35
19. Using a Boyle's Law approach
Converting pressure to absolute:
99 fsw x 1 atm = 3 atm ambient ==> 4 ata
33 fsw
132 fsw x 1 atm = 4 atm ambient ==> 5 ata
33 fsw
Substituting into Boyle's Law
(4 ata) (25 min) = (5 ata) )x min)
x = 20 minutes
20. a. Volume of cylinder is independent of gas content. So, the cylinder will hold the same amount of nitrox as air; 80 cubic feet at 3000 psig.
b. Working with absolute pressures:
Pabsolute1 = 3000 psi + 14.7 psi = 3014.7 psi:
Pabsolute2 = 2400 psi + 14.7 psi = 2414.7 psi
Now, determine volume available at 2400 psi on the gauge:
80 ft3 = x
3014.7 2414.7
X = 64.1ft3
The volume of gas available from a fixed volume cylinder will be directly proportional to the absolute pressure. Converting gauge pressure to absolute:
c. Convert gauge pressure to absolute:
1800 psig + 14.7 psig = 1814.7 psia
3000 psig + 14.7 psig = 3014.7 psia
2475 psig + 14.7 psig = 2489.7 psia
For 80 cubic foot cylinder:
80 ft3 = Volume
3014.7 psia 1814.7 psia
Volume = 48.15 ft3
For 71.6 cubic foot cylinder:
71.6 ft3 = Volume
2489.7 psia 1814.7 psia
Volume = 52.18 ft3
21. a. The buoyant force of the object is equal to volume of sea water displaced = 4 cubic foot
4 ft3 x 64 lbs = 256 lbs
ft3
Since the object weighs less than its buoyant force, it will float.
Density of the object = mass/volume:
Density = 96 lbs / 4 ft3 = 24 lbs / ft3
Amount submerged is the ratio of the density of the object and the density of the fluid:
Submerged = 24 lbs / ft3 = 37.5 %
64 lbs/ ft3
b. This requires balancing weight (downward force) with buoyancy (upwards force).
The total of weight (diver equipment plus lead) needed to hover in salt water is equivalent to the buoyant force = 198 lbs + 18 lbs = 216 lbs
To
hover, the volume of water displaced by the diver must exert an upward buoyant
force equal the total weight of the diver plus gear (downward force). This is
the upward buoyant force exerted by the displaced volume of seawater (density =
64 lb./cubic foot) that weighs 216 pounds.
Determine Volume of diver: (Divers use weight and mass as equivalent terms)
Density = Weight / Volume
Rearranging:
Volume = Weight / Density
Volume = 216 lbs = 3.38 ft3
64 lb/ft3
The upward buoyant force from fresh water (density 62.4 lb./ cubic foot) that
the diver would displace:
3.38 ft3 x 62.4 lb = 210.9 lbs ===> 211 lbs
ft3
Applying force arrows:
Fresh water buoyant force = 211 lbs![]()
Diver
weight
= 198 lbs ![]()
Net
=
13
lbs ![]()
So, the diver that was
comfortable with eighteen pounds of lead on the weight belt in seawater must
remove 5 pounds (for a total of 13 lb.) from the weight belt to dive in fresh
water. The difference in density between fresh and seawater is the reason why
different amounts of weight must be used when diving in different environments.
When moving from fresh to seawater (with the same equipment configuration),
divers must add weight. When moving from seawater to less dense fresh water,
divers should remove weight.
Thirteen pounds cannot be worn symmetrically on the weight belt, so in this case, the diver would choose 14 pounds of lead to be a tiny bit heavy, but balanced.
22. a. Determine buoyancy force (weight of displaced water):
2.0 ft3 x 64 lb = 128 lbs
ft3
Determine amount of lift needed to raise the anchor
Weight of anchor = 524 lbs ![]()
Buoyancy = 128 lbs![]()
Net Force = 396 lbs ![]()
To lift the anchor, 396 pounds of lift must be supplied. This is equivalent to the a water volume of:
396 lbs x 1 ft3 = 6.19 ft3
64 lbs
Determine absolute pressure at depth
66 fsw x 1 atm = 2 atm ===> 3 ata
33 fsw
Use Boyle's Law to determine surface equivalent
(1 ata) (Vsurface) = (6.19 ft3 )(3 ata)
Vsurface = 18.57 ft3
b. Determine Volume of concrete block using definition of density:
150 lbs/ ft3 = 2460 lbs / Volume
Volume = 16.4 ft3
Determine Weight of displaced water (buoyant force):
16.4 ft3 x 64 lbs = 1,049.6 ==> 1050 lbs
ft3
Forces diagram:
Weight of the block = 2460 lbs ![]()
Buoyant force = 1050 lbs ![]()
Net Force = 1410 lbs
![]()
So, need 1410 pounds of lift to raise the object
If each device can only supply 75% of capacity, then need to supply
1410 lbs = 1880 lbs of lift
0.75
The number of 55 gal drums needed is:
1880 lbs = 4.31 ==> 5 drums
436 lbs/drum
The volume of water needed to supply lifting force:
1880 lbs x ft3 = 29.4 ft3
64 lbs
The absolute pressure at depth
86 fsw x 1 atm = 2.6 atm ===> 3.6 ata
33 fsw
The volume of at the surface needed to supply lifting volume
(1 atm) Vsurface) = (3.6 ata) (29.4 ft3)
Vsurface = 105.8 ft3
The number of 80's needed
105.8 ft3 = 1.32 tanks ==> 2 tanks
80 ft3 /tank
23. Since salt water is more dense than fresh water, an equal volume of sea water will weigh more (have more buoyant force). So, for the same immersed object, less lift will be needed in salt water than in fresh water. So, equipment that can manage a lift in fresh water should be able to accomplish same lift in sea water.
Volume of the object:
V = pi r2 l
V = (3.14) (2.5 ft)2 (50 ft)
V = 981.8 ft3
Weight of the object using definition of density:
150 lbs/ ft3 = weight / 981.8 ft3
weight = 147,270 lbs
Weight of water displaced in fresh water:
981.8 ft3 x 62.4 lbs/ ft3 = 61,264.32 lbs
Weight of water displaced in salt water:
981.8 ft3 x 64 lbs/ ft3 = 62,835.2 lbs
Force Needed to lift in fresh water:
Weight of object = 147,270 lbs ![]()
Buoyant force = 61,264 lbs ![]()
Net Force = 86,006 lbs ![]() ==> Need at least 86,006 pounds of lift to overcome in-water weight
==> Need at least 86,006 pounds of lift to overcome in-water weight
Force Needed to in salt water:
Weight of object = 147,270 lbs ![]()
Buoyant force = 62,835 lbs ![]()
Net Force = 84,435 lbs ![]() ==> Need at least 84,435 pounds of lift to overcome in-water weight
==> Need at least 84,435 pounds of lift to overcome in-water weight
24. a. A mole (the molecular mass of a substance expressed in grams) of any gas occupies 22.4 liters at STP (standard temperature and pressure: 0 oC (273 K); 1 ata). Since one mole of dry gas at STP occupies 22.4 liters, the density of each pure substance:
Density O2 = 31.998 g/mole x 1 mole/22.4 L = 1.428 g/L
Density N2 = 28.014 g/mole x 1 mole/22.4 L = 1.251 g/L
Density He = 4.00 g/mole x 1 mole/22.4 L = 0.178 g/L
The density of a mix is the sum of the components; at STP:
O2 0.21 x 1.428 g/L = 0.300 g/L
N2 0.29 x 1.251 g/L = 0.363 g/L
He 0.50 x 0.178 g/L = 0.089 g/L
Density Mix = 0.752 g/L
b. Absolute pressure at 225 fsw
225 fsw x 1 atm = 6.81 atm ==> 7.81 ata
33 fsw
Since density is directly proportional to pressure:
Trimix Ddepth = 0.752 g/L x 7.81 = 5.87 g/L
Oxygen Ddepth = 1.296 g/L x 7.81 = 10.12 g/L
25. a. Each gas is treated separately:
Final volume is 200 l + 300 L = 500 l
Using Boyle's Law
For Oxygen:
(200 l) (200 mm) = (500 l ) (P)
P = 80 mm
For nitrogen:
(300 l) (100 mm) = (500 l) (P)
P = 60 mm
b. Using Henry's Law
Total pressure = 80 mm + 60 mm = 140 mm Hg
26. For an ideal gas:
PV =nRT ==> P = nRT/V
P = (80 moles) (0.0821 l-atm/deg mole) (298 K) / (11.3 l)
P = 173.21 ata
P psi = 173.21 ata x 14.7 psi /ata = 2546.2 psi
Pgauge = 2546. 2 psi - 14.7 psi = 2531.5 psig
For a real gas (using Van derWaal's)
(P + an2/V2) (V -nb) = nRT
Rearranging:
P = nRT
- n2a
(V-nb)
V2
P = (80 moles) (0,0821 l-atm/deg-mole) (298 K) - (80 moles)2 (0.03412 atm/mole2)
(11.3 l - 80(0.0237 l/mole)) (11.3 l)2
P = 208.12 atm - 1.71 atm = 206.4 atm
P psi = 206.4 atm x 14.7 psi/atm = 3,034.2 psi
P gauge = 3024.2 psi - 14.7psi = 3,019,5 psi
Since there is a significant difference in calculated pressures, it would be unwise to use ideal equations for helium containing mixes.
Go To: Home About "Harris" Articles Slides War Stories Editorials Links Fini
About
The Author:
Larry "Harris" Taylor, Ph.D. is a biochemist and Diving Safety Coordinator at the University of Michigan. He has authored more than 200 scuba related articles. His personal dive library (See Alert Diver, Mar/Apr, 1997, p. 54) is considered one of the best recreational sources of information In North America.
All rights reserved.
Use of these articles for personal or organizational profit is specifically denied.
These articles may be used for not-for-profit diving education