 |
Dive Physics
Problem Set 2 by Larry "Harris" Taylor. Ph.D.
Diving Safety Coordinator University of Michigan Ann Arbor, Michigan |
 |
 |
Dive Physics
Problem Set 2 by Larry "Harris" Taylor. Ph.D.
Diving Safety Coordinator University of Michigan Ann Arbor, Michigan |
 |
These problems were designed to CHALLENGE UNDERSTANDING.
Go To: Home About "Harris" Articles Slides War Stories Editorials Links Fini
1a.
A sprinter runs a 100 m in 9.6 sec. What is the average speed of the
sprinter in miles per hour?
b.
A long distance runner runs a 4 minute mile. What is the average speed of
the runner in miles per hour?
c.
One measure of physical fitness is the ability to swim 400 yards in 12
minutes. What is the average speed required to meet this requirement in miles
per hour?
2.
You are standing on the edge of a river. You notice that a piece of
floating debris takes 92 seconds to travel 100 feet. What is the speed of the
river in meters/sec and miles per hour?
3.
Your underwater camera has focus adjustments calibrated in feet. You have
measured the distance from the camera to the object to be photographed as
exactly 6 feet. At what distance do you set the camera focus?
4.
In the ancient kingdom of Utopia, a racquetball-like game was the Royal
sport. As such, the unit of measurement for area was the Jox. (The area of the
royal racquetball court that measured 30 feet x 60 feet). If a football field
measures 132 feet x 300 feet, how many jox are there on a football field?
5. In the original series of Star Trek episode "City on the Edge of Forever," Captain Kirk and Mr. Spock were stranded in the US circa 1935. When Mr. Spock was requested to start working on a solution, he asked for 5 pounds of platinum. Given that the 1935 price of gold was $35.00 per TROY ounce (precious metals use the Troy ounce which has 12 ounces per Troy pound) and the average price for platinum is 8 times the price of gold, how long would it take our intrepid Captain Kirk working at 15 cents an hour (and assuming all his earnings went towards the purchase of platinum) to obtain the funds necessary to obtain this amount of platinum for Mr. Spock?
6.
The temperature outside is 52 oF.
The dew point is 56 oF.
Will there be visible fog from your breath when you exhale?
7
a.
Cumulus clouds form when water vapor ascending from the surface is cooled
by the atmosphere. Given that the dew point drops about 4.5 oF
for every one thousand feet of altitude, what is the height at the base of a
cumulus cloud when the surface temperature is 70 oF
and the surface dew point is 50 oF?
b.
It is now 9:00 pm on a warm cloudless fall evening. The temperature is 68
oF.
The dew point is 58 oF.
You notice the temperature is dropping 2 oF
per hour. There is a full eclipse of the moon that will reach totality at
midnight. Based on dew point, will the eclipse be visible, or will it be
obscured by fog?
8.
How much heat is needed to raise the temperature of 100 grams of water
(specific heat 1.0 cal/g oC)
10 oC?
9.
The "average" swimmer doing the crawl stroke uses about 9
kcal/minute. Based purely on heat content, how long must the swimmer swim to
"burn-up" the equivalent of a "fast-food" lunch consisting
of one super large jumbo burger (605 kcal), a package of French fries (214 kcal)
and a large milk shake (332 kcal)?
10.
The "average hypothermia victim," requires about 60 kcal to
raise the body temperature 1 oF (W. Fogery, HYPOTHERMIA: DEATH BY EXPOSURE, p. 54).
A liter of water at 110 oF
(about the maximum temperature that people can tolerate to drink) contributes
about 17 kcal. If a conscious hypothermia patient (core temperature 90 oF) were to drink this heated water, how much water
would be necessary to raise the core temperature to 98.6 oF? Based on this amount of fluid, is relying solely on
warm drinks to restore body temperature a viable first aid procedure?
11
a.
Hypothermia is a severe problem in many diving environments.
In an attempt to increase tolerance to cold water, some divers are using
other gases (instead of air) to inflate their dry suits.
In other words, they are using inert gases, not air, as an insulator. Based on
the thermal data listed below, is this a good idea?
Hint: Using heat capacities, estimate the amount of heat needed to raise 100 g of each, 10 oC
| Substance | Cp (cal/g oC |
| Air | 0.3439 |
| Argon | 0.1252 |
| Helium | 4.9680 |
b. It is known that CO2
has a smaller thermal conductivity than air. Since CO2
is much cheaper than Argon, would CO2
be a good choice for a diver to use as an insulating gas in a dry suit?
12.
Assuming Ideal gas behavior (i.e., Boyle's Law), is air more dense at 78
fsw than the surface? If so, by how much?
13.
Assuming an average density of air to be 0.0012 g/cc and the density of
mercury to be 13.6 g/cc, estimate the height of an air barometer that represents
one atmosphere of pressure? Express this number in meters and in miles.
14.
You have purchased a brand new depth gauge for your trip to Bonaire. Upon
suiting up in Bonaire, you notice that the gauge was calibrated in feet fresh
water. Assuming the gauge HAS NO INHERENT ERRORS and that all the errors are in
the difference between the calibrated gauge and the actual water pressure, what
does the gauge read at 100 fsw?
15.
You have been commissioned by Indiana Jones to help recover a sacred gold
headpiece covered with emeralds. Indy's map indicates that the headpiece is
located at the bottom of a high mountain lake. By lead line, you measure that
the bottom lies at 93 feet. Unfortunately, while you were determining the bottom
depth, one of the bearers set fire to the supplies. The altitude adjusting dive
computers, the high altitude dive tables, and Bruce Weinke's superb book on
altitude diving have been destroyed by the fire. All that remains is a National
Bureau of Standard's calibrated barometer and some charred fragments of a US
Navy Dive Table. Since you do not wish for Indy to get bent, you must determine
the equivalent sea level ocean depth to utilize the US Navy Dive Tables. The
barometer reads 546 mm Hg. (Fortunately, you remember the specific gravity of Hg
is 13.6 and the specific gravity of fresh water is 1.00)
Charred
Remains of Dive Table:
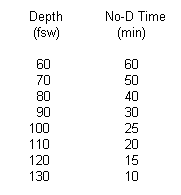
a. What will Indy's oil filled depth
gauge read on the bottom?
b. What is the equivalent sea level
ocean depth?
c. How long does Indy have to find and
recover the headpiece without entering a decompression situation?
16.
You are the diving officer for the new colony on the planet Xandor. The
density of the Xandor sea is 1.47 g/cc. The atmospheric pressure on Xandor at
sea level is 680 mm Hg (density Hg = 13.6 g/cc). Your habitat floats on the
Xandor sea. You wish to explore the area directly underneath your habitat. The
sea bottom there is at a depth of 67 feet.
a. On the bottom, what is absolute
pressure in Earth & Xandor atmospheres?
b. What will your earth calibrated
depth gauge read at 67 feet in the Xandor sea?
c. What earth depth should you use on
your earth decompression tables to determine your decompression obligation?
d. On earth, your absolute air consumption
rate was 50 psig/ata-min for a standard aluminum 80 cylinder. Using this size
cylinder, (and starting ascent at 1000 psig) how long will this cylinder last at
depth? Assume that the pressure reading upon reaching the bottom was 2700 psig.
e. In your diving equipment, you weigh
225 pounds. You required 12 pounds of lead to hover while diving in earth's
ocean. How much lead will be needed to dive in the sea of Xandor?
17a.
To avoid oxygen toxicity problems, it is desired that the partial pressure of
oxygen not exceed 1.6 ata under normal (non-stressed) diving conditions. Based
on this oxygen partial pressure, what is the maximum depth for a dive on pure
oxygen and compressed air
dive (air = 21 % O2)?
b. Under stress (work loads, cold,
deep, etc.) it is recommended that the maximum pO2
not exceed 1.4 ata. Based on oxygen toxicity concern, what is the maximum depth
for a dive on pure oxygen and compressed air?
18.
Your scuba cylinder is filled outdoors during an ice storm and the tank
is equilibrated in a 45 oF
water bath to a pressure of 3000 psig. What will your pressure be before diving
if you allow the cylinder to equilibrate to a water temperature of 78 oF?
19.
Assuming your air supply lasts 25 minutes at 99 fsw, If all other factors
were equal, how long would this air supply last at 132 fsw?
20 a. A scuba cylinder holds 80 cubic feet of air at 3000 psig. How much Nitrox would the cylinder hold at 3000 psig?
b. A scuba cylinder holds 80 cubic
feet of air at 3000 psig. How much air is available at 2400 psig?
c. Which has more volume of gas
available:
1) an 80
cu. ft. tank (3000 psig) at 1800 psig, or
2) a
71.6 cu. ft. tank (2475 psig) at 1800 psig?
21
a.
A 4 cubic foot device weighing 96 pounds is placed in sea water. How much of
this object is submerged?
b. A diver with equipment weighs 198
pounds. In salt water, the diver requires 18 pounds to hover. How
much lead should the diver wear in fresh water?
22.
a.
A 524 pound anchor with a volume of 2 cubic feet is lying on a hard flat bottom
in 66 feet of sea water. How much lift is needed to raise the anchor? What is
the surface equivalent volume of air needed to supply this lift?
b. How many 80 ft3 scuba cylinders and 55 gallon drums (436 pounds of
lift each) fitted with over-expansion vents would be needed to raise a
reinforced concrete block that had a surface weight of 2,460 pounds (density of
concrete = 150 lbs/cubic foot) lying at 86 fsw on a hard flat bottom? Assume
that there is significant drag on the lifting devices such that each device can
only lift 75% of theoretical capacity.
23.
Assume that you wish to lift two cylinders 5 feet in diameter by 50 feet
long of concrete (density = 150 lbs per cubic foot; V = pi r2 l). One cylinder is in the ocean, the other in a near-by lake. Since
you wish to save time and money (renting large lifting devices is expensive),
you calculate your needs for both lifts based on lifting the object from the
nearby lake. Can you do this? Why or Why not?
24
a.
Calculate the density at STP of a mixture containing 21% O2,
50 % He, and 29 % N2. The
molecular weight of O2 is
31.998; the molecular weight of N2
is 28.014, and the molecular weight of He is 4.00.
b. What is the density of this mix at
225 fsw. Compare this to the density of air at this depth (STP density of air =
1.296 g/l)?
25
a.
A 200 l flask contains oxygen at 200 mm Hg absolute pressure. A 300 ml flask contains
nitrogen at 100 mm Hg absolute pressure. The two flasks are connected. What is the final
partial pressure of each gas?
b. What is the total pressure?
26.
What is the pressure calculated for an 80 ft3
cylinder filled approximately 80% of its capacity with Helium. The physical
volume of an aluminum "80" is about 11.3 liters. Calculate this
pressure using a value of n = 80 moles at a temperature of 25 oC
( 298 K) for both the Ideal and Real (Van Der Waals') gas law equation.
Given: Universal gas constant, R =
0.0821 l-ata/deg-mole
a = 0.03412 l2
-ata/mole2
b = 0.02370 l/m
Based on this calculation, are Ideal gas laws suitable for estimating concentrations of breathing gas mixes that contain Helium?
Go To: Home About "Harris" Articles Slides War Stories Editorials Links Fini
About
The Author:
Larry
"Harris" Taylor, Ph.D. is a biochemist and Diving Safety Coordinator
at the University of Michigan. He has authored more than 100 scuba related
articles. His personal dive library (See Alert Diver, Mar/Apr, 1997, p. 54) is
considered one of the best recreational sources of information In North America.
All rights reserved.
Use of these articles for personal or organizational profit is specifically denied.
These articles may be used for not-for-profit diving education