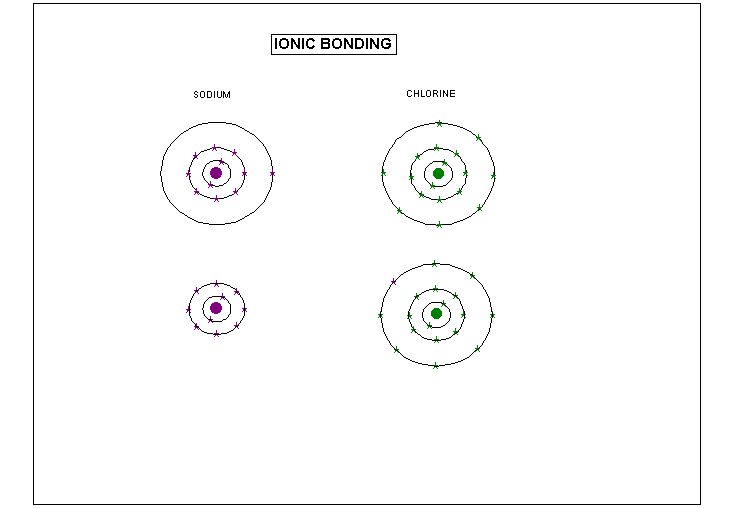
In Ionic bonds, electrons are lost by one atom
and gained by another. When an atom loses an electron, it gains a positive
charge because it has lost some negative charge from the loss of the electron.
When and atom gains an electron, it gains a negative charge because it
gains some negative charge from the added electron. When an element
loses or gains electrons it is called an ION. Because opposites attract
positive and negative atoms stick together, or bond, a neutral compound
is formed.

In the above example, chlorine gave up an electron and sodium got rid of an electron. The two combine together to form NaCl, more commonly known as table salt.
Anything between 2 metal or a metal and a non-metal is an ionic bond. Looking at the periodic table, you can see that the metals are located on the left and the non metals on the right.
An everyday example of ionic bonding is the rusting of a car. Metals tend to readily lose their electrons because they only have one or two in their outer energy level. The iron in the car's frame bonds with the oxygen in the air causing a chemical reaction forming rust.