
What are chemical bonds?
Chemical bonds are attractions that hold two
or more atoms together.
Is the periodic table helpful in
determining bonding?
Because atoms make up everything in the world,
they must also make up the elements on the periodic table as well.
The periodic table plays an important role in determining the bonding abilities
of the elements. In order to show the importance of the periodic
table, first let's see how it is set up.
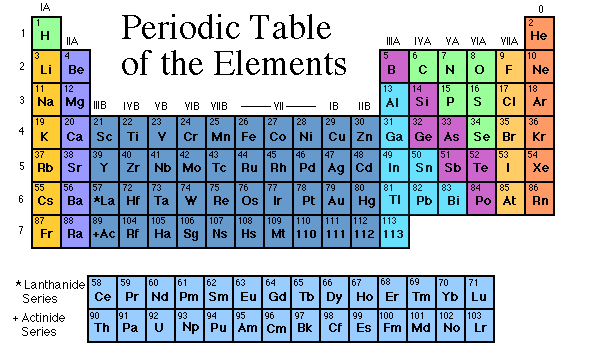
How is the periodic table organized?
First, the periodic table is broken down into
metals and non-metals. To the far right of the table their is a line,
like a staircase, that separates the metals and the nonmetals. The
line is after the elements B, Si, As, Te, and At. Everything to the
right of the line are non-metals and everything to the left of this line
are metals.
Next, the periodic table is broken down into
periods (vertical rows) and groups (the columns). The elements on
the periodic table are then classified according to both the periods and
the groups.
How are elements organized in periods?
Elements are organized according to atomic number
in the periods. The ATOMIC NUMBER of an element means the number
of protons in the nucleus of that element's atoms. As you move from
left to right and down the columns, the atomic number gets bigger.
That means the number of protons in the elements also increases.
How are elements organized in groups?
Elements in the columns are organized by similar
physical and chemical properties. For instance, similar melting and
boiling points and most importantly the same reactivity.
What is reactivity?
Electrons are the determiners of reactivity.
Atoms are only stable, or happy, when they have enough electrons to fill
the outer most energy level. They will react with other elements
in order to get these full, stable outer levels. Most atoms are stable
when they have 8 electrons in their outer energy level. An element
can gain, lose, or share electrons when in order to be happy. All
the elements in group 8, called the noble gases, are special because
they already have full energy levels. Because they are already happy
they are not reactive all.
How does this relate to the groups
of elements?
The groups on the periodic table are an excellent
resource to find out which elements lose electrons, which elements gain
electrons, and which elements share electrons.
In all the tall groups, designated with the letter
A, it is easy to determine which elements gain or lose electrons, thanks
to how the periodic table is set up.
Group A1 all have one extra electron in their
outer level.
Group A2 all have 2 extra electrons in their
outer level.
Group A3 all have, you guessed it, 3 extra electrons
Group A4 has 4 extra electrons
Group A5 has 5 extra electrons
Group A6 has 6 extra electrons
Group A7 has 7 extra electrons.
Now What?
We know that all the elements want is to be stable
and happy by having a full energy level, so in order for that to
happen we have to do something about the extra electrons. The only
problem is that most of the elements are really pretty lazy! They
want to be happy but don't want to do alot of work to get this happiness.
Elements in groups 1 have only one electron in
there outer level. It would be much easier for them to get rid of
1 electron than to try and find 7 others to have a full outer level.
So they kick one out to be happy!
Elements in group 7, however, have 7 electrons
in their outer level. It would be way to much work for them to try
and get rid of 7 electrons, so they kidnap one from someone
else. This is called IONIC BONDING
because the atoms are giving or taking electrons from one another.
If it is to hard to steal or kick an electron
out atoms sometimes share the electrons between them. This is called
COVALENT BONDING.
Some Good Links:
periodic table
Go back to home