


Everything in the world is made up of atoms.
They are the building blocks of all things.
Every atom consists of protons (+),
electrons (-),
and neutrons (o)
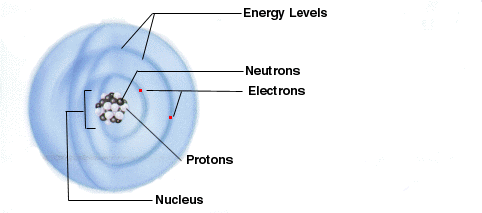
Many energy levels surround the nucleus in
rings, forming what scientists call shells. You can picture them best if
you think of the layers of and onion. You can keep peeling multiple layers
of skin and eventually you will get to the center, or nucleus.
Each energy level can only hold a certain number of electrons. For example, the first level holds only 2, the second level holds 8, and the third level holds 18. The electron configuration determines where these electrons will go.
Most atoms, besides hydrogen, are stable when
they have 8 electrons in their outermost energy level. Hydrogen has only
one energy level, therefore it is stable with only 2 electrons.
Because atoms want to be stable, they can
lose, gain or share electrons with other atoms to achieve 8 electrons in
their outer level. Atoms do this by a process call Chemical Bonding.