Biomarkers of Aging
Richard A. Miller
October, 2001
Originally prepared for SAGE-KE.
URL: http://sageke.sciencemag.org/cgi/content/full/sageke;2001/1/pe2
As a subgenre of biogerontology, biomarker research has developed a thoroughly tarnished reputation, and those of us who believe that biomarker studies should be a major focus in experimental aging research must now try to prove to our doubting colleagues that under the tarnish lies true metal, rather than more layers of tarnish. The difficulties have, I think, four root causes: (a) mathematical problems that have led some researchers to circular arguments [see (1, 2) for a discussion of this point]; (b) philosophical problems, typified by the objection that biomarkers cannot measure aging because there is no such thing as aging; (c) buyer's remorse associated with a substantial, highly publicized governmental investment in biomarker research that proved to be less fruitful than its sponsors had hoped; and (d) the Missouri syndrome, in which skeptics justifiably adopt a "show-me" attitude, looking for evidence that biomarker research will clear some of the hurdles looming between its proponents and the goal line. These difficulties are compounded by matters of definition: Various experts in this area, and various neophytes, too, have often proposed formal definitions of biomarkers that are, to differing degrees, in conflict or frankly unsatisfactory.
Outside of gerontology, biomarkers play an important, acknowledged role as surrogate measures of processes or traits that are themselves difficult to measure. A typical case comes from endocrine physiology: Although it is difficult to measure accurately the mean level of blood sugar in a diabetic patient over an extended period of time, tests for glycated hemoglobin are simple to perform, and their results convey much of the required information. The validation of hemoglobin glycation as a surrogate for integrated blood glucose levels required careful comparison of both kinds of data under a range of conditions in multiple test subjects; once accomplished, however, it allowed endocrinologists to use glycation values to meet a range of research and clinical objectives. It is not necessary (or possible) for glycation values to convey all of the information that might be obtained by constant monitoring of glucose levels. Indeed, there are situations in which glycation data are not a useful substitute for glucose values (for example, after an erythropoietic stress or in the context of a glucose tolerance test). Stock market averages, tumor grading scales, summary scores from personality inventories, bond ratings, and Apgar scores all convey useful information in convenient packages, even though none of these surrogates can possibly encompass all of the information about the system--the economy, disease, brain, bond, or infant--that the index is designed to summarize.
Examples of this kind put gerontologists into an awkward position, because many modern biogerontologists do not believe that there is such a thing as aging; in their minds, there is no core process for which a biomarker can serve as a surrogate. Many authorities, for which Masoro and Holliday can stand as examples (3, 4), view aging as a more-or-less parallel progression of multiple degenerative processes, some of which lead to cancer, others to alterations of connective tissue, still others to neurodegeneration, and yet others to muscle wasting. From this viewpoint, processes of this sort could potentially exert overlapping effects on several cell types and organ systems, but none of them serves as a "master clock" that synchronizes these deleterious changes. This argument constitutes objection (b) above: the assertion that a biomarker of aging is as fanciful a construct as the weight of a goblin, in that both goblins and aging are imaginary items for which metrics cannot be expected or condoned.
The anti-biomarker camp (or this faction of it, anyway) is heartened by deconstructions (1) of previous attempts to develop mathematical composites of age-sensitive traits that could be mistaken for quantifiable indices of "generalized aging." The critics of linear regression and principal components approaches to biomarker construction [responsible for objection (a) above] argue, I think convincingly, that evidence for some correlation among different age-sensitive traits does not constitute proof that these traits are suitable indices for some unmeasured (and perhaps unmeasurable) "rate of aging." Previous attempts to develop batteries of biomarker assays have also stumbled over statistical obstacles, including failure to control for age-independent differences among subjects in the traits of interest: Low muscle strength in an old man may reflect rapid muscle aging, life-long weakness, or both. In my view, though, these critics jump too quickly from "has not been done" to "cannot be done." At the heart of their argument is the allegation that biomarkers of aging cannot be developed because "there is no single rate of aging . . . different functions change over time at different rates and for different reasons, and none can be considered a marker of some basic process of aging" [(1), p. 31]. These critics may be right, but the question is too important to be decided by edict rather than by experimentation.
We can safely leave objection (c) to historians and social psychologists and focus the rest of this essay on objection (d): the "biomarker challenge." Those of us who think that there is indeed an aging process--a process that regulates, albeit imprecisely, the pace of a wide range of late life changes in multiple organs, cells, and extracellular domains--should be able to show skeptics that this process has a rate that can be measured. If we are successful, we will have constructed a tool of great value for interventional gerontology. Navigation was not quite impossible before the invention of clock and sextant, nor is applied gerontology quite impossible today. But it is easy to see how the invention and (more important) validation of a measuring device for aging rate and biological age could simplify and greatly hasten the testing of interventions (and possibly mutations or allele combinations) alleged to alter aging and thereby delay or decelerate late life illness.
To begin, I'd like to propose a definition of a biomarker of aging. To serve as a biomarker, a trait would need to meet three criteria: (i) it should predict the outcome of a wide range of age-sensitive tests in multiple physiological domains, in an age-coherent way, and do so better than chronological age; (ii) it should predict remaining longevity at an age at which 90% of the population is still alive; and (iii) its measurement should not alter either life expectancy or the outcome of subsequent tests of other age-sensitive traits.
Item (i) is the core of the definition. Let's say that one has evidence that a particular T cell subset, the CD4M cell type, tends to rise on average with age, and one now wishes to see whether CD4M levels can serve as biomarkers for aging in mice. If one could document that mice with relatively high CD4M levels also, at a given chronological age, had relatively weak muscles, advanced cataracts, patterns of liver gene expression characteristic of older mice, high levels of collagen cross-linking, and poor nerve conduction velocity, these observations would buttress the case for CD4M cells as a biomarker of aging (that is, an index of some fundamental process that influences the pace of age-sensitive changes in multiple, apparently independent, cells and organ systems). It is highly unlikely that any candidate biomarker would show strong correlations with all age-sensitive traits, because most traits are regulated by factors (such as genetic alleles, dietary or social factors, and stochastic events) that are unrelated to aging, and in many cases the (hypothesized) effects of aging will be too small to be detectable. I know of no age-sensitive traits that have passed the first of these tests, but then again I know of none that have been evaluated in an appropriately systematic fashion.
Of course, many age-sensitive tests show statistical associations with one another simply because they change with age. For example, people with cataracts frequently are found to have limited exercise endurance or poor immune responses, in part because people with cataracts are usually old people. The key to evaluating potential biomarkers is to determine to what extent these associations hold up after statistical adjustment for age effects (that is, after removing the portion of the correlation that is due to age alone). The simplest way to accomplish this task is to make the measurements on individuals of the same chronological age. In cases where that is not possible, multivariate statistics provide useful approaches for analysis of more complex age-structured data sets. The most useful information is likely to be obtained in middle age, when the aging process has already had an effect, but when few subjects have been lost through death and the complex effects of serious illness and its treatment need not yet be considered for most subjects.
Item (ii)--predicting remaining longevity--is included as a way of tying the biomarker validation process to the current "gold standard" for interventional gerontology: all-cause mortality rates. Despite the weaknesses of using either mean or maximal life-span as oversimplified indices of aging rate, survival curves are still, deservedly, a critical part of the argument that a diet or a gene slows down aging. It is easy to construct scenarios in which an intervention alters life-span but does so independently of any effect on aging (such as low-fat diets for the atheroma-prone, low-salt diets for the hypertensive, or low-bullet environments for those attracted to violent companions). One of the difficulties associated with satisfying criterion (ii) occurs when experiments are conducted with any group of test animals in which nearly all die of a single cause, such as autoimmune disease, mammary cancer, or renal failure. Such a situation presents serious problems, confounding effects on a specific disease process with possible effects mediated via alteration of aging rate. Demonstration that a candidate biomarker can predict longevity in a group of genetically heterogeneous mice, in which individuals die of different diseases, would add strong support to the argument that the biomarker provides an index of aging rate, because only by slowing aging per se would it be possible to retard so many forms of lethal illness.
Tests that predict life expectancy only very close to the time of death are less informative, because it is hard to exclude the idea that the outcome of the test may have been influenced by the same illness or preclinical syndrome that led to death. Nearly all attempts to show that immunological tests predict life-span in humans can be challenged on this basis, because in all cases except one (5), the follow-up period (2 to 4 years) was a very small fraction of the total life-span.
Item (iii)--that biomarker measurement should not alter either life expectancy or the outcome of subsequent tests of other age-sensitive traits--is included with a view toward both experimental validation and clinical applicability. Tests that involve the death of the subject, such as tests that depend on tissue from internal organs, cannot be incorporated into protocols that will have clinical utility and, furthermore, cannot be validated by showing that the test predicts remaining life-span. Tests conducted on tissues produced from euthanized animals, though not themselves useful as biomarkers, could in principle play a role in a program designed to validate more suitable candidate biomarkers of aging. A study of the value of peripheral blood T cell function as a biomarker of aging could, for example, make use of data showing which of the functional assays were best associated with age-sensitive measures of bone or heart structure and function.
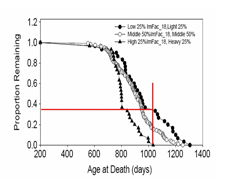
My laboratory group, together with collaborators, has embarked on a program dedicated to evaluating age-sensitive changes in immune status as potential biomarkers of aging in mice. We have produced some relevant evidence, which might charitably be characterized as promising but not yet conclusive. For example, we have shown that each of four different T cell subsets measured at 18 months of age in genetically heterogeneous mice whose median survival is about 27 months can predict remaining longevity with P values <0.003 or better (6). Further, we have used principal component analysis to combine T cell subset values into more informative indices. The largest such factor (that is, the combination of T cell subset values that captures the most variation among the animals tested) was found to correlate strongly and consistently with those individual T cell subset measurements that are altered by aging, thus showing that the factor could be interpreted as an index of immunological age. Our unpublished calculations have shown that this first principal factor, measured at 18 months of age, is a significant (P < 0.0001) predictor of longevity, although it accounted for only a small proportion (6%) of the variation in life-span. Interestingly, the same factor was a significant predictor of life-span in subgroups of mice dying, respectively, of breast cancer, fibrosarcoma, or lymphoma, as well as in mice dying of all other causes combined. These findings are consistent with the hypothesis that age-sensitive changes in T cell subset patterns either reflect or contribute to vulnerability to a wide range of late-life illnesses. Work is in progress to assess whether these or other measures of T cell status correlate with age-sensitive changes in cell, tissue, or organ function; preliminary evidence for correlation with tests of muscle strength has already been published (7).
The discipline of gerontobiomarkerology faces a long uphill climb, with too little to show, so far, for the many miles behind us. But if, as suggested by caloric restriction data (8) and more recent work on long-lived mutant mice (9), there really is an aging process that synchronizes the vast array of age-dependent changes on individual organ systems, it may be possible to develop a way to estimate the rate of this process even without a detailed understanding of the biochemical pathways that link aging to its many signs and symptoms. There are thousands of reports of traits that change with age in adults, but very few researchers have begun to tackle the next key step in biomarker validation: documenting that the biomarker measurements do a better job than mere calendar age in predicting the results of other age-sensitive tests and health outcomes. The development of a battery of validated indices of biological aging will require careful planning, substantial secure funding, and collaboration among experts in physiology and biometry. Getting public or private money for such testing is very difficult, and there is no organized program to develop such a biomarker battery at this time. The successful identification of biomarkers would constitute a landmark in our understanding of the aging process and provide invaluable tools for screening potential anti-aging interventions that could be of major benefit to preventive medicine and public health.
October 3, 2001
References Cited
1. P. T. Costa, R. R. McCrae, in Handbook of Physiology. Section 11: Aging, E. J. Masoro, Ed. (Oxford Univ. Press, New York, 1995), pp. 25-36.
2. P. T. Costa, R. R. McCrae, Measures and markers of biological aging: "a great clamoring . . . of fleeting significance." Arch. Gerontol. Geriatr. 7, 211-214 (1988).
3. E. J. Masoro, in Handbook of Physiology. Section 11: Aging, E. J. Masoro, Ed. (Oxford University Press, New York, 1995), chap. 1.
4. R. Holliday, Understanding Ageing (Cambridge Univ. Press, Cambridge, 1995).
5. S. J. Wayne, R. L. Rhyne, P. J. Garry, J. S. Goodwin, Cell-mediated immunity as a predictor of morbidity and mortality in subjects over 60. J. Gerontol. Med. Sci. 45, M45-M48 (1990).
6. R. A. Miller, Biomarkers of aging: prediction of longevity using age-sensitive T cell subset determinations in a middle-aged, genetically heterogeneous mouse population. J. Gerontol. A Biol. Sci. Med. Sci. 56A, B180-B186 (2001).
7. R. A. Miller, F. Bookstein, J. H. van der Meulen, S. Engle, J. Kim, L. Mullins, J. Faulkner, Candidate biomarkers of aging: age-sensitive indices of immune and muscle function co-vary in genetically heterogeneous mice. J. Gerontol. Biol. Sci. 52A, B39-B47 (1996).
8. B. P. Yu, in Handbook of Physiology. Section 11: Aging, E. J. Masoro, Ed. (Oxford Univ. Press, New York, 1995), chap. 23.
9. K. Flurkey, J. Papaconstantinou, R. A. Miller, D. E. Harrison, Life span extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc. Natl. Acad. Sci. U.S.A. 98, 6736-6741 (2001).

A small mouse and a large fly
T cell subsets and weight predict lifespan in HET mice (J. Harper)