Oxygen:
Cylinders, Concentrations, Grades, and Labels
by
Larry
"Harris" Taylor, Ph.D.
This material is copyrighted and all rights retained
by the author. This article is made available as a service to the diving
community by the author and may be distributed for any non-commercial or
Not-For-Profit use.
All
rights reserved.
Go
To: Home
About
"Harris" Articles
Slides
War Stories
Editorials
Links
Fini
Jump
to: USP Grades
Cylinders Label Legal Litigation
Prescriptions Praxair
SB-107 Recommendation
The use of oxygen, particularly in recreational gas mixes, is often a topic for
debate, posturing, and rhetoric. This article summarizes the various terms and usage associated with
compressed gas cylinders containing oxygen for human respiration and medical
procedures.
Oxygen
Oxygen is a chemical element that exists
in the atmosphere as a stable molecule containing two atoms (O2).
The element has an atomic weight of 16; the molecular weight is 32.
Oxygen is one of the most abundant
chemical elements on earth. Like most gases, oxygen is colorless, odorless, and
tasteless. It is chemically reactive and readily combines with a variety of
materials. Although by itself oxygen is not combustible, the ease with which it
reacts with other materials can lead to fires and explosions if improperly handled. Mixtures above 21-23.45 % O2 are considered
oxygen-enriched atmospheres by the Compressed Gas Association (CGA) and the
National Fire Protection Association (NFPA). The risk of fire/explosion
increases with increasing oxygen partial pressure and/or concentration. Handling
oxygen-enriched atmospheres requires specialized equipment and training.
Oxygen
is essential for life. The body uses chemical reactions based on oxygen to
generate heat and chemical energy. This process, called metabolism, keeps us
alive. The oxygen in the breathing gas must be maintained within certain limits.
Too little oxygen (hypoxia) can be fatal. Too much oxygen (hyperoxia) can lead
to CNS (Central Nervous System) oxygen toxicity with the possibility of seizures
at depth or a more long term development, whole body or pulmonary oxygen
toxicity. These
toxicity concerns for the oxygen provider are discussed here.
Almost
all the oxygen (as well as nitrogen and argon) manufactured in the US is
prepared from the fractional distillation of liquid air. In this process, air is
chilled to a liquid state and then gradually warmed so that oxygen, nitrogen and
argon (which have different boiling points) can be separately collected
and packaged. Additionally, a small
amount of oxygen is prepared from the electrolysis of water or from solid-state
chemical reactions.
USP
Entry for Oxygen
The United States Pharmacopoeia
(USP) is a listing (compendium) of drugs licensed for use in the United States
with the analytical standards necessary to establish purity suitable for human
use applied to each of the listed drugs. Drugs sold
for human consumption in the US must meet or exceed the purity standards listed in the USP.
Drugs meeting these requirements must include a designation on their label that
demonstrates compliance with standards listed within the USP; typically, this is
shown as the initials USP immediately following the name of the drug. Many nations
have their own Pharmacopoeia to list and specify requirements for drugs legally sold within their borders.
To be labeled
Oxygen, USP, the gas cylinder must contain a documented minimum of 99.0 percent oxygen by
volume and be odor free. The label must also specify whether the oxygen gas was
produced by the fractional distillation of air (also called air-liquefaction
process) or other means. If produced from liquid air, then the cylinder contents
may be labeled USP without an analysis for either carbon dioxide (CO2) or carbon
monoxide (CO).
It is illegal
in the US to sell non-USP drugs (including oxygen) for human consumption.
Oxygen Grades
The US Department of Transportation (DOT)
classifies oxygen as a non-flammable
compressed gas. The Compressed Gas
Association (CGA) has designated various grades of oxygen based on analysis of
the contents of an oxygen cylinder. The table below, unless otherwise
specified, lists possible contaminant components in parts per million (ppm) and
the
analytical standards for each of the various defined grades. A blank entry means
that there is no specification or test required for the listed component in the
appropriate grade column.
Grade A is the
minimum requirement for USP oxygen. Grade E is commonly called aviator's grade.
Grade
of Oxygen Gas
|
Content
|
A
|
B
|
C
|
D
|
E
|
F
|
G
|
|
Oxygen
Min. % (mole)
|
99.0
|
99.5
|
99.5
|
99.5
|
99.6
|
99.995
|
99.5
|
|
Water
(v/v)
|
|
|
50
|
6.6
|
8
|
1.0
|
2
|
|
Dew
Point (oF)
|
|
|
-54.5
|
-82
|
-80
|
-105
|
-97
|
|
Methane
|
|
|
|
50
|
|
|
|
|
Nitrogen
|
|
|
|
|
|
|
100
|
|
Ethylene
|
|
|
|
0.4
|
|
|
|
|
Acetylene
|
|
|
|
0.1
|
|
|
|
|
Carbon
Dioxide
|
300
|
|
|
10
|
|
1.0
|
5
|
|
Carbon
Monoxide
|
10
|
|
|
|
|
1.0
|
|
|
Total
Hydrocarbons (as methane)
|
|
|
|
|
50
|
1.0
|
25
|
|
Ethane
& Other Hydrocarbons
|
|
|
|
6
|
|
|
|
|
Nitrous
Oxide
|
|
|
|
4
|
|
0.1
|
2
|
|
Halocarbons
|
|
|
|
2
|
|
|
|
|
Solvents
|
|
|
|
0.2
|
|
|
In addition,
there are a variety of terms used in the lay community that have been used
to describe various grades of oxygen. These sometimes are specific to a local
community or state. One of he most
intense discussions commonly encountered in discussing oxygen use for diving centers
on the term "welding gas." This is primarily only of historical
interest.
Many moons ago,
gas supply vendors typically maintained two separate storage facilities for
oxygen: " welding or industrial or technical" (a non-USP, or illegal to sell
for human consumption label) variety and gas intended for
human use in respirators or medical procedures (a USP label). Since most gas supply vendors have decided that it is simply not
cost-effective to store separate grades of medical gases, almost all oxygen
sold in the US is a USP grade (meets USP requirements for human consumption).
However, there are distinct differences in how the cylinders are filled. (see
below). So, the debate as to whether to use a non-USP welding gas or a USP
medical grade oxygen for diving is mostly irrelevant since most vendors are now
filling all cylinders with USP gas.
Typically, when
filling an incoming oxygen cylinder, the fill station operator opens the
cylinder valve and "sniffs" the valve opening. If acetylene is smelled
(an indication of improper shut down procedures in a welding operation), the
cylinder is set aside to be cleaned at some later time (to remove the odor
from acetylene and thus prevent a potential explosion from an acetylene-oxygen
mix within the cylinder). If no acetylene is smelled, then a whip is
connected to the cylinder and the "welding" cylinder is filled with USP oxygen.
If the vendor
fill station operator sees that a cylinder is labeled as a medical or emergency
oxygen cylinder, or intended for human respiration, then the cylinder is evacuated (see below) and filled with USP oxygen.
So, the difference between a
modern US "welding" oxygen or "medical" or "respiratory"
oxygen
cylinder is not the quality of the oxygen gas used to fill the cylinder,
but the manner in which the cylinder is filled (and the legal consequences of
inappropriate fill procedures) and the FDA labeling requirements.
Today in the
US:
Most
welding or
Industrial grade oxygen cylinders are filled with USP oxygen without an evacuation-between-fills
step.
Respiratory
grade oxygen cylinders are filled with USP oxygen with an evacuation-between-fills
step.
Medical grade
oxygen cylinders are filled with USP oxygen with an evacuation-between-fills step
(Some states
differentiate between a respiratory and medical grade. Typically, the cylinder
contents are the exactly the same, but the label is different. Historically, this was
done to avoid prescription requirements for emergency/rescue organizations using pure
oxygen respirators.)
Aviation grade
oxygen cylinders are filled with USP oxygen that has undergone additional drying
steps with an evacuation-between-fills step. The low quantity of water is a precaution against
oxygen line freezing that might occur with higher water content at the chilled
temperatures found at altitude. (Typically, "regulator freeze-up" is a
result of chilled water vapor within the gas mix condensing and then freezing
... a chunk of ice may block gas flow or interfere with mechanical operation of
valve/regulator mechanisms. So, lowering water vapor content of a gas mixture lessens the likelihood of a
"freeze-up.")
Grade 4.5
oxygen is USP oxygen that has a purity greater than 99.995 % oxygen
Grade 5 (or
"five nines") oxygen is USP oxygen that is certified 99.999 % pure. It is sometimes called
research grade. This is the highest purity of oxygen manufactured in
the US and is typically found only in top-end chemical research facilities. Its
non-research use is associated with specialty welding of titanium and titanium
alloys for the nuclear or aerospace industry.
For divers, the
best oxygen available for deco or oxygen-enriched air breathing mixes is
aviation grade oxygen.
For emergency-only (DAN) cylinders, the only legally
acceptable gas is medical grade oxygen supplied by an FDA licensed facility. (see legal
stuff, below).
The
Cylinders
The
use of oxygen, particularly in recreational gas mixes, is often a topic for
debate, posturing, and rhetoric. This table summarizes the various types of
compressed gas cylinders containing oxygen for human respiration and medical
procedures.
|
Cylinder
Size
|
Oxygen Capacity
|
Service Pressure
|
Cylinder Length
|
Cylinder O.D.
|
Cylinder Weight
|
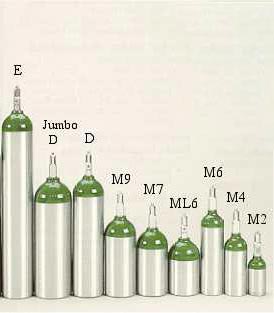
|
| |
CU. - LITER
|
PSI - BAR
|
IN. - CM
|
IN. - MM
|
LBS. - KG
|
|
MM
|
122 - 3455
|
2216 - 153
|
35.75 - 90.8
|
8.0 - 203
|
38.6 - 17.55
|
|
M60
|
61.4 - 1738
|
2216 - 153
|
23.0 - 58.4
|
7.25 - 184
|
21.7 - 9.86
|
|
E
|
24 - 680
|
2015 - 139
|
25.63 - 65.1
|
4.38 - 111
|
7.9 - 3.58
|
|
Jumbo D
|
22.6 - 640
|
2015 - 139
|
16.3 - 41.4
|
5.25 - 133
|
8.1 - 3.68
|
|
D
|
15 - 425
|
2015 - 139
|
16.51 - 41.9
|
4.38 - 111
|
5.3 - 2.41
|
|
M9
|
9 - 255
|
2015 - 139
|
11.88 - 27.6
|
4.38 - 111
|
3.7 - 1.69
|
|
M7
|
7 - 198
|
2015 - 139
|
9.18 -23.3
|
4.38 - 111
|
3.3 - 1.48
|
|
ML6
|
6 - 165
|
2015 - 139
|
7.68 - 19.5
|
4.38 - 111
|
2.9 - 1.29
|
|
M6
|
6 - 165
|
2216 - 153
|
11.59 - 29.4
|
3.2 - 81
|
2.2 - 1.0
|
|
M4
|
4 - 113
|
2216 - 153
|
8.4 - 21.3
|
3.2 - 81
|
1.6 - .74
|
|
M2
|
1.4 - 40
|
2216 - 153
|
5.37 - 13.6
|
2.5 - 63.5
|
.74 - .34
|
The Label
The label from
one of my E (~650 liter) cylinders is shown below (large enough to read the
print).

The label designates
the following:
Oxygen,
Compressed USP
This cylinder contains compressed oxygen gas that meets the purity standards for
human use as defined by the United States Pharmacopoeia
(USP).
UN 1072
The DOT chemical compound identification number is 1072. This is a reference to the
Material Safety Data Sheet (MSDS) which lists physical properties, special handling
precautions, chemical reactivity, health hazards, waste disposal and
transportation requirements for the cylinder contents. (US OSHA regulations
mandate that all chemicals sold in the US be accompanied by a MSDS to
facilitate safety when handling purchased chemical materials.)
Yellow
Diamond
This is a
reference symbol established by the National Fire Protection Agency (NFPA)
to quickly identify the nature of potential hazards associated with the chemical
contents of a container. The yellow color implies the contents may contribute to
substantial releases of energy. This symbol contains the name of the
chemical, Oxygen, and identifies it as a combustion hazard (the fire symbol) with
a relative danger of 2 (of 4 possible). The relative danger of 2 suggests
a potential of
violent combustion, an increased fire threat typically without immediate detonation.
Contents
This blank
allows the vendor to specify volume of contents contained within the cylinder at
the time of filling.
Warning
This is this
the legal consumer product safety warning. Notice that this label allows filling
of emergency-only cylinders without prescription,
Produced by
Air Liquefaction
Identifies the method
of manufacturer that exempts contents from required-for-USP-label carbon dioxide or carbon
monoxide analysis.
Warning
Specific
hazards associated with the chemical nature of oxygen. The Chemical Abstracts
Service (CAS, a chemical organization that abstracts all published chemical
journals, as well as assigning unique numbers to all chemicals known as they are
discovered) identification number for oxygen (CAS 778244-7) is also provided as
a reference to the chemical and physical properties of the cylinder contents.
The caution to use in accordance to the MSDS refers to a reference document (the
Oxygen MSDS furnished by the manufacturer) that lists known concerns about the safety issues associated with
handling the contents of the cylinder.
Do
Not Remove
FDA requires that drugs always be properly labeled such that the
contents are readily identifiable.
The Vendor
The label must contain the source of the drug (in this case, Ann Arbor Welding
Supply) since this FDA licensed facility will have records required by FDA Good
Manufacturing Practices on the handling of the gas contained in the cylinder.
The Legal
Stuff
Before
mentioning the FDA regulations for compressed medical gases, it should be
pointed out that any business in the United States that has employees must meet both Federal and State
compliance with respect to Occupational Health and Safety Regulations ( OSHA).
Although originally intended for chemical laboratories, an additional program
called "chemical hygiene" is observed by many business operations.
"Chemical Hygiene" can be interpreted as having safety data (typically as MSDS (Manufacturing
Safety Data Sheets)) for all chemicals (including compressed gases) on the
premises. In addition, the employer must document that all employees have been briefed
on specific hazards and procedures associated with every chemical present at the
business location. To
document OSHA compliance, various agencies will supply checklists to assist
employers in meeting these guidelines. Failure
to meet OSHA guidelines can be quite expensive since OSHA has the power to issue
daily fines and close a business until compliance is documented. Wise business
owners will seek legal advice to understand steps necessary for compliance with
local and Federal OSHA regulations.
The US Federal Drug Administration (FDA)
classifies oxygen as a compressed medical gas (CMG)
and, as such, it must
be handled in accordance with the appropriate federal guidelines. Use of oxygen
as a CMG is covered by a minimum of 2 separate
federal documents: Title
21 CFR Parts 200-211 (drug labeling act) and the FDA Medical Gases Guidelines
( as a PDF
file from the FDA )
Basically,
most "civilized" nations require that drugs
intended for human use be prepared under guidelines that have been called "Good Manufacturing Practice (GMP)." The GMP is a set of protocols
supervised in the US by the FDA that
require all steps, from initial manufacture to dispensing of the
drug to the consumer, document the purity of the material being distributed.
So, each phase of drug distribution must maintain records of drug analysis and
handling consistent with the standards defined for the specific drug in the USP and GMP guidelines (analogous to a legal "chain of
evidence"). Compressed medical gas cylinders can be legally filled only at
a FDA inspected and licensed facility.
For medical
gases, including oxygen, the primary GMP protocol that affects divers is the
transfilling (moving gas between storage and use cylinders) requirement. The FDA Guidelines
for this step state:
Requirements
for drug containers:
Section 211.94(c) requires that drug product containers be clean.
Section 211.94(d) requires that standards or specifications, methods of
testing, and, where indicated, methods of cleaning be written and followed for
drug product containers.
Guidance
One factor to consider regarding the above requirements is the possible
presence of foreign gas residues in CMG cylinders before filling. An acceptable
method of assuring that cylinders do not contain foreign gas residues is to pull
a vacuum on each cylinder equal to 25 or more inches of mercury prior to filling
with the CMG. (Cryogenic vessels are seldom completely emptied and need not be
evacuated before filling.)
Most medical
cylinders and emergency response respirators using oxygen are used repeatedly; often the same cylinder may be used in a variety
of locations with differing practices of handling cylinders. The requirement of always
evacuating a cylinder, regardless of cylinder pressure, is a step intended
to minimize contamination that might occur from "back-filling" of a cylinder
improperly connected to a gas cascade system, or left open to the atmosphere for
extended periods of time. It also prevents the accumulation of possible
contaminants from repeated fills from a compromised source. The primary distinction of oxygen cylinders intended
for medical (emergency applications are also medical procedures) procedures and
respirators and
all other uses is
this evacuation step between fills.
The
Compressed Gas Association publication P-2.5, Transfilling of High Pressure
Gaseous Oxygen To Be Used For Respiration, states that the transfilling station
should consist of "a supply cylinder unit, a receiving cylinder
unit, a cylinder evacuation unit, a gas transfer control unit, and detailed
written procedures."
So, the cost of
filling an oxygen cylinder intended for medical or emergency-only or for human
respirators service is
primarily not
a function of the gas cost, (300 - 700 liter consumer cylinders are
typically filled for the same price), but the price for the required documented analysis
associated at each
step of
distribution and the equipment necessary at each step to evacuate a 100% oxygen
cylinder to a significant vacuum prior to refilling. There is also the
assurance that the cylinder contents have been validated at each handling step.
While many
divers might consider this evacuation-between-fills step unnecessary, it is considered by the FDA to be an
essential requirement for medical grade gasses. While rare, there have been
injuries and fatalities associated with failure to evacuate oxygen cylinders
between fills. As such, the evacuation step is considered by the FDA to be absolutely essential
in filling cylinders intended for medical (emergency) use.
The
FDA does prosecute the inappropriate filling of oxygen cylinders by unlicensed
facilities.
From the
Medical Gas Guidelines Appendix:
QUESTION: What are the penalties for violating the Act?
ANSWER: If found guilty of violating a provision of the Act, an officer or
employee of the firm may be imprisoned for up to one year and/or fined up to
$100,000 ($250,000 if death occurs) for each violation. If the prohibited act
was committed with intent to defraud or mislead or if an officer or employee is
convicted a second time under the Act, the offense is punishable by a fine of
$250,000 to $500,000 and imprisonment of up to three years for each violation.
The firm can also be fined the same amounts. (Note: The limits of the amount of
fine that can be imposed were raised by the Comprehensive Crime Control Act of
1984, for all criminal violations for which the basic Federal law imposes a
prison sentence of six months or more.)
One interesting feature of the law as written is
that as long as the oxygen gas is USP, it may be used for mixing all sorts of
concentrations of oxygen containing breathing mixes. However, the transfilling
of 100% oxygen into cylinders intended for medical or emergency-only
cylinders requires that the FDA license the transfilling station and that
all cylinders filled with compressed medical gas be filled according to FDA
specified GMP procedures.
Accident Litigation
It is pretty much a
given in the US that a dive accident will lead to litigation. In such an event,
the plaintiff will explore as many as possible avenues to convince a
jury that the defendant was not a responsible, caring person ... someone whose
disregard of accepted safety standards was the proximate cause of an injury or
death. If oxygen was on the site and used in accident management, then the
nature of the cylinder contents becomes a legal matter. Using a FDA licensed facility
as a medical gas vendor facilitates defense; use
of FDA non-compliant cylinders, irrespective of the actual gas purity,
potentially provides the
plaintiff (and jury) with documentation that the defendant was acting outside the law.
Prescriptions
The historical
basis of prescriptions for oxygen is discussed in the article Rx:Oxygen.
Historically, there have been 4 types of oxygen cylinders exempt from
prescription requirements. These are cylinders used only for:
1.
emergency use by trained personnel
2.
chemical/medical research or education
3.
extremely limited capacity
4.
private or commercial aviation
However, medical insurance providers, either
third party or the federal government, pay for most non-hospital use of oxygen
cylinders. This means the oxygen supply vendor is often
audited to document amounts of gas distributed and the conditions of distribution.
Regardless of the nature of an emergency-only cylinder, it is simply easier and
more convenient to provide the oxygen supplier with a prescription. Besides,
many commercial vendors will simply not fill an oxygen cylinder for individuals without a prescription.
This
is how I handle the situation.
Each
year (Michigan limits oxygen prescriptions to one year), I have a physician write me a prescription for "oxygen to be used in
dive accident management or education." This satisfies the vendor's need
for documents when audited and allows me to fill my cylinders. I keep a photocopy
of my personal prescription for oxygen in my DAN O2 case, in case there is
ever a need to refill a used oxygen cylinder while traveling.
DAN
Agreement With Praxair
The
Diver's Alert Network (DAN) has made arrangements with a commercial
medical gas supplier, Praxair, to assist divers in filling their DAN oxygen
cylinders.
CGA SB-107
In 1992, the
Compressed Gas Association issued a special warning about oxygen cylinders to
the diving community; special bulletin 107 (SB-107). This was a result of
several catastrophic failures during refill operations of oxygen cylinders that
had been immersed in ocean water. These failures included several fatalities of
fill station operators. It has now been documented that water can enter a
cylinder via the regulator when the purge is depressed, regardless of cylinder
gas pressure. The combination of seawater with high oxygen concentration can
destroy the integrity of a steel cylinder within 30 to 60 days. As such, the CGA has recommended:
1. if
possible, do not submerge cylinders containing high concentrations of oxygen
2.
cascade system working pressure should never drop below 2 x the
at-depth pressure.
3. never
transfill oxygen (or any other gas) cylinders without the owner's consent.
So, What
to do?
Personally, I
get my oxygen-enriched mixtures from my local dive shop (that uses aviation
grade oxygen in their gas mixing operations). However, I will only fill
my DAN O2 cylinders (and additional D & E cylinders used in
teaching DAN O2 classes) from an FDA licensed transfilling station. To me, a reasonable and prudent person would always
fill their emergency response cylinders at an FDA licensed facility.
Another
guideline I use is that if the cylinder has a scuba regulator compatible valve,
then I fill the cylinder at a dive shop. However, any oxygen cylinders with a
medical regulator valve (uses the pin-indexing system) are only filled at a FDA
licensed facility.
References
-----,
COMPRESSED MEDICAL GASES GUIDELINE, U.S. Public Health Service, Food & Drug
Administration, Rockville, MD. 1989, 24 pages.
From CGA
G-4:
Oxygen, 1987, 16 pages.
G-4.3:
Commodity Specification for Oxygen, 1988, 12 pages.
P.2.5
Transfilling of High Pressure Gaseous Oxygen To Be Used For Respiration, 13
pages, 1992.
SB-7:
Rupture of Oxygen Cylinders in the Diving Industry, 1980, 2 pages.
From NFPA
-----,
"Medical Gases," Chapter 7 in NFPA Fire Protection Handbook, p. 5-49 -
5-57.
Top
Jump
to: Oxygen USP Grades
Cylinders Label Legal Litigation
Prescriptions Praxair
Go
To: Home
About
"Harris" Articles
Slides
War Stories
Editorials
Links
Fini
About The
Author:
Larry
"Harris" Taylor, Ph.D. is a biochemist and Dive Safety Coordinator at the
University of Michigan. He has authored more than 200 scuba related articles.
His personal dive library (See Alert Diver, Mar/Apr, 1997, p. 54) is considered
one of the best recreational sources of information in North America.
Copyright 2001-2024 by Larry "Harris"
Taylor
All
rights reserved.
Use
of these articles for personal or organizational profit is specifically denied.
These
articles may be used for not-for-profit diving education

