by
Larry "Harris" Taylor, Ph.D.
This is an
electronic reprint of an article that appeared in Great Lakes Diving (Feb/Mar.
1995, p. 18, 20-21). This material is copyrighted. All rights retained by the
author. This article is made available as a service to the diving community by
the author and may be distributed for any non-commercial or Not-For-Profit
use.
All rights
reserved.
Go To: Home About "Harris" Articles Slides War Stories Editorials Links Fini
Diving is fun! Being cold is not! Perhaps the greatest
deterrent to diving is the cold. Cold is a physiological stressor. With the
possible exception of the death of a loved one or relationship, there is perhaps
no greater stress than the cold. It is possible to dive in cold environments and
enjoy it. All that is needed is a bit of understanding of the nature of heat and
cold (the absence of heat), the manner in which we delay the inevitable loss of
heat, and a little bit of coin to purchase the necessary
protection.
Heat
Heat is thermal energy; the sum of the kinetic energies for
all the random movements of all molecules contained within a substance. All substances can be thought of as heat
reservoirs. The amount of heat within this reservoir is subject to change. Food
intake or external heat sources such as the sun, extremely hot water, or radiant
heaters can add heat to the body. Heat loss from a variety of mechanisms
(discussed below) can remove heat. The body, unless overwhelmed by the
environment or disease, usually keeps our internal heat reservoirs at an
appropriate level.
Heat can be envisioned as a "fluid" in motion. It ALWAYS moves
from the warmer object to the colder object until the temperatures of the
objects touching each other are at the same temperature. This means that when
you enter Lake Superior, the heat "fluid" leaves your body in an attempt to
raise the temperature of Lake Superior to your body temperature. Since the mass
(size of the heat reservoir) of Lake Superior is so large compared to the mass
of your body, the movement of heat from you to the water will cause no
significant rise in temperature of Lake Superior, but the attempt to warm the
entire lake will drain significant amounts of heat from you. This heat drain can
result in lowering of the body's temperature (hypothermia). Severe hypothermia
can be life threatening.
A measure of the rate at which heat flows from the body is
termed thermal conductivity. Some values of interest to the diver are listed
below:
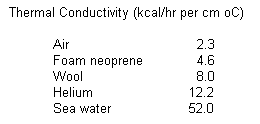
This means
that a diver loses heat into the water approximately 25 times faster than into
dry, still air at the same temperature. So, the obvious means to stay warm is to
stay dry.
A diver loses heat in several ways:
Conduction: the primary
heat loss mechanism in diving. This is the loss of thermal energy by direct
contact between substances; a molecular transfer of energy. Heat moves from the
warm diver to the cooler water. Water is denser than air (has more molecules per
unit volume); thus more molecular collisions per unit volume occur with the
result of more heat transfer. Water will remove heat from a diver 25 times
faster than dry still air of the same temperature. If the diver is breathing gas
at a temperature below body temperature, the diver will lose heat as the
breathing gas is warmed by body heat. The denser the breathing gas mixture
(i.e., the deeper the dive), the greater the heat loss due to respiration. Note
that heat loss in respiration is related to the density of the breathing mix.
This means helium mixes (much less dense than air) will be less heat robbing
than air at the same depth.
Convection: is associated with conduction. As a
volume of cooler fluid in contact with a warm body is heated, it expands and
circulates away from the contact point. The area vacated by the warmer fluid is
filled with cooler fluid and more heat transfer occurs. Convection increases the
conductive heat loss. A wet suit functions, in part, by limiting the conductive
and convective heat loss by restricting the flow of the volume of water
immediately surrounding the diver.
Evaporation: the loss of
heat associated with a change in state of liquid water to water vapor. A
significant amount of energy is required to change liquid water to water vapor.
A diver breathes a very dry gas. The lungs need a humidified gas. As the diver
breathes, water is evaporated along the respiratory tract to humidify the
breathing mixture. This can result in significant heat loss from the diver.
Additionally, much heat can be lost from the evaporation of water on a diver's
clothing (for example, a wet suit) on a breezy day.
Radiation: the loss of
heat energy by direct emission of heat energy (infrared radiation). Although
this means of losing heat is of critical importance in the vacuum of outer space
(minimal air molecules for the process of conduction and convection to occur),
heat loss to the submerged recreational diver is small.
The loss of heat from a diver immersed in cold water CANNOT
BE PREVENTED. It can, however, be slowed. The purpose of insulation,
generally gas trapped in some physical matrix (like nitrogen bubbles in a wet
suit or air in the dry suit underwear), is to slow the loss of heat from the
diver to the water by imposing a physical barrier. Thus, heat must move through
the insulation on its way from the diver to the water. The better the
insulation, the longer it will take the body heat of the diver to move into the
water.
Wet suit compression associated with the increased pressure of
descent not only decreases buoyancy, it reduces the volume of the protective
insulation (gas trapped in rubber material of the wet suit). This is why wet
suits offer less thermal protection in deeper water.
The Layer Concept Of Thermal Protection
Heat ALWAYS
moves from the warmer body to the colder material. This process cannot be
prevented. It can, however, be slowed enough to allow the diver time to enjoy a
cold water dive in relative comfort. The rate of thermal loss will depend on
diver size, the temperature of the water, the amount of time in the water,
amount of physical exertion and the nature of the insulation system employed.
Modern thermal protection systems are composed of three distinct layers. They
are the "wicking" layer, the insulation layer, and the protective outer
layer.
The "Wicking" Layer
Since wet
skin conducts heat away from the body much faster than dry skin, it seems
obvious that a prime consideration in thermal protection is to keep the skin
dry. This is accomplished with the use of a layer of synthetic material.
Commonly used materials are called polypro, polypropylene, or Capilene. These
materials are "hydrophobic" (they will not absorb water), thus water from the
skin is "wicked" away from the skin, through the synthetic material into the
next layer away from the skin. This keeps the skin dry and heat is retained much
longer.
Anyone who has used these materials will note that they soon
"acquire" a distinct odor. This is best removed by washing in a detergent made
especially for breathable fabrics (available from outdoor outfitters.) It is
important to examine the material after washing for odor. The garment MUST BE
odor free. If you can smell detergent, then the detergent trapped within the
material will absorb water and increase thermal loss. So, if odor is noticed,
continue rewashing in warm water (no detergent) until no odor is noticed. Then,
tumble dry with minimal heat. Insuring a clean, dry "wicking" layer is the first
line of defense in protecting the diver from thermal loss. (Synthetic insulation
is laundered in the same manner.)
Remember, the purpose of the layer next to the skin is to keep
the skin dry and minimize the accelerated heat loss associated with the direct
transfer of heat from body to water. This means the first layer MUST BE a
synthetic material. ALL natural fibers absorb water. Eventually, this absorption
of water reaches a point where the material becomes an excellent heat conductor.
Cotton is a particular No, No! Cotton is a fantastic summer fiber. This is
because cotton is a polar material and thus, readily absorbs water. A little bit
of air movement and the evaporation of this absorbed water provides a
comfortable cooling effect. This same cooling effect so appropriate during the
summer, will dramatically speed heat loss in cold environments. Fogery, in DEATH
BY EXPOSURE suggests that wearing cotton jeans during the winter in outdoor
activities can significantly reduce survival chances. So, to stay warm in cold
climates, keep the first layer next to the skin a synthetic fiber that will not
absorb water.
The
Insulation Layer
The insulation layer provides the primary resistance to
thermal loss. Its purpose is to slow the unavoidable loss of heat from the body
to the cold-water environment. The amount of insulation used and the material
employed will depend upon a number of factors. Although there are a number of
guidelines available, individual experience is still the best determination of
insulation used for a given dive. There is NO UNIVERSAL insulation package that
will suit all divers, over all types of diving, over the large temperature
ranges one finds in the Great Lakes. Active divers will have several different
types of insulation packages to mix and match to suit diving conditions and
personal comfort levels. Remember that thermal comfort is an individual matter
and it is possible to see a number of different systems on the same dive. Divers
should determine thermal comfort on an individual basis.
The insulation in dry suit underwear is air trapped within the
matrix of the insulation garment. Insulation effectiveness varies with the
ability of the underwear to hold air. Basically, thermal insulation for dry
suits falls into four types: wooly bears, open cell foam, type b marine
thinsulate, and radiant barriers. We will examine each.
Wooly bears can be
comprised of either natural (wool), synthetic (polyester, for example) or
blends. They are called wooly bears because the insulation looks like fur. The
fuzzy side is worn next to the skin. This underwear provides decent insulation
at a reasonable price. It is often the first underwear purchased by a novice dry
suit diver. When purchasing, pay attention to the number of fibers per unit
area. This determines the insulation value (more fibers, more insulation, better
heat retention). Avoid simply comparing weight. The weight comes primarily from
the backing material and may give a false impression of the insulation
value.
The underwear may shed. This fuzz can get into valves and
compromise their function. The underwear will compress a bit at depth and lose
some effectiveness. The major disadvantage of this type of material is that it
loses thermal resistance rapidly when wet.
Open cell foam is very warm
when dry. Its major advantage is that it does not compress at depth. It tends to
be a little less flexible than other underwear types. Its major disadvantage is
that the open cells provide a heat conduit when wet. Although an excellent
insulator when dry, this underwear rapidly loses effectiveness when
wet.
Type B marine
thinsulate is, perhaps, the best available
material. It is practically incompressible and absorbs no water. Thus it retains
the majority (80 %) of its insulation capability, even when totally submerged.
It is called thinsulate because the amount of material needed to provide the
same amount of insulation is much less than when compared to wool or down. Thus,
thinsulate garments can provide thermal protection while minimizing bulk. It is
important to verify that the thinsulate used is type b. This is marine
thinsulate. This material is non-compressible and extremely water resistant.
Other thinsulates are not as effective in wet environments.
Radiant
barrier garments prevent heat loss by
radiation, These are extremely efficient insulators in the vacuum of space.
However, heat loss by radiation is minimal in divers. To be effective in diving,
these insulators must be combined with other materials to slow down heat loss by
conduction or convection.
Thermal Comparisons
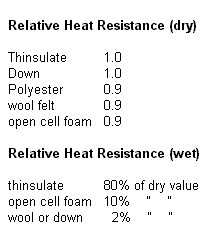
The Protection Layer
The modern
(shell) dry suit has no inherent thermal protective properties. Its sole purpose
is to protect the inner layers. As long as the outer layer protects the
insulation from water, the insulation layer can work at its maximum potential.
This protection function is most important. Divers should read and understand
dry suit manufacturers maintenance guidelines. Proper maintenance ensures the
dry suit will perform to its specifications.
Conclusion
With a bit of knowledge and the appropriate equipment, it is possible to not only survive cold waters, but to enjoy the experience.
Thermal protection is illustrated in the slide set on DrySuits
About The
Author:
Larry "Harris" Taylor, Ph.D. is a biochemist and Diving Safety Coordinator at the University of Michigan. He has authored more than 200 scuba related articles. His personal dive library (See Alert Diver, Mar/Apr, 1997, p. 54) is considered one of the best recreational sources of information In North America.
All rights reserved.
Use of these articles for personal or organizational profit is specifically denied.
These articles may be used for not-for-profit diving education