KubarychGroup
Surface-bound Water Sensor
As we showed for the first time, transition metal carbonyl vibrations are remarkably sensitive to the presence of water. They are also able to tell us the nature of the water they sense: is the water bulk-like or confined? We found the influence of the protein on the water dynamics to be spatially heterogeneous.
Sensitivity to Large Angular Jumps
Based on observed differences between H2O and D2O, he hypothesized that a major component of the dynamics we observed could be attributed to large angular jumps of water molecules solvating the vibrational probe. Hence, the protein’s role in modifying the water’s dynamics is to inhibit these angular jumps. Our picture is consistent with explicit protein-water molecular dynamics simulations carried out by the group of Damien Laage at the Ecole Normale Supérieure in Paris.
Using two different lysozymes (hen, blue, and human, yellow), we can probe different locations of nearly identical proteins without making any mutations.
Protein Hydration
The hydrophobic effect is essential for biological function since it is the main driving force for protein folding, membrane formation and interactions between biomolecules. Much is known about the thermodynamics (relative energies) associated with the hydrophobicity, but it is comparatively more challenging to probe the dynamics (i.e. motion, flexibility and fluctuations) experimentally due to the small number of hydrating water molecules relative to the vast amount of bulk solvent.
Using a novel protein labeling approach, where we covalently attach very strong IR probes site-specifically to the protein surface, we are able to sense both protein and water dynamics through their influence on the vibrational lifetime and spectral diffusion time scales of the probe chromophore.
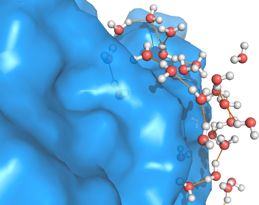
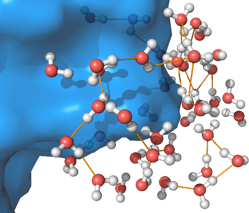
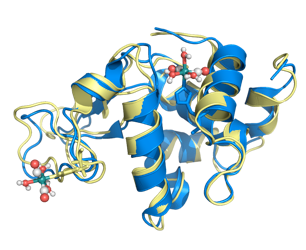




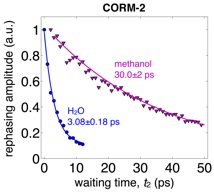
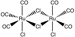
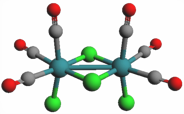
The small molecule CORM-2 (a so-called “carbon monoxide releasing molecule”) relaxes an order of magnitude faster in water than in methanol.
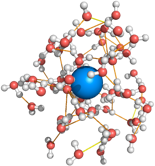
Small hydrophobes preserve water’s hydrogen bonding network, whereas large molecules tend to force the sacrifice of hydrogen bonds. Topologically heterogeneous molecules like proteins can appear both small and large depending on the location.

