Playing leap-frog with Elephants:
emi, ltd. and CT scanner competition in the 1970s
Revision history:
August-September, 1989 (Mitchell)
January-June 1990 (Smith and Mitchell)
April 23, 1991 (Mitchell)
December 1994: Minor revision
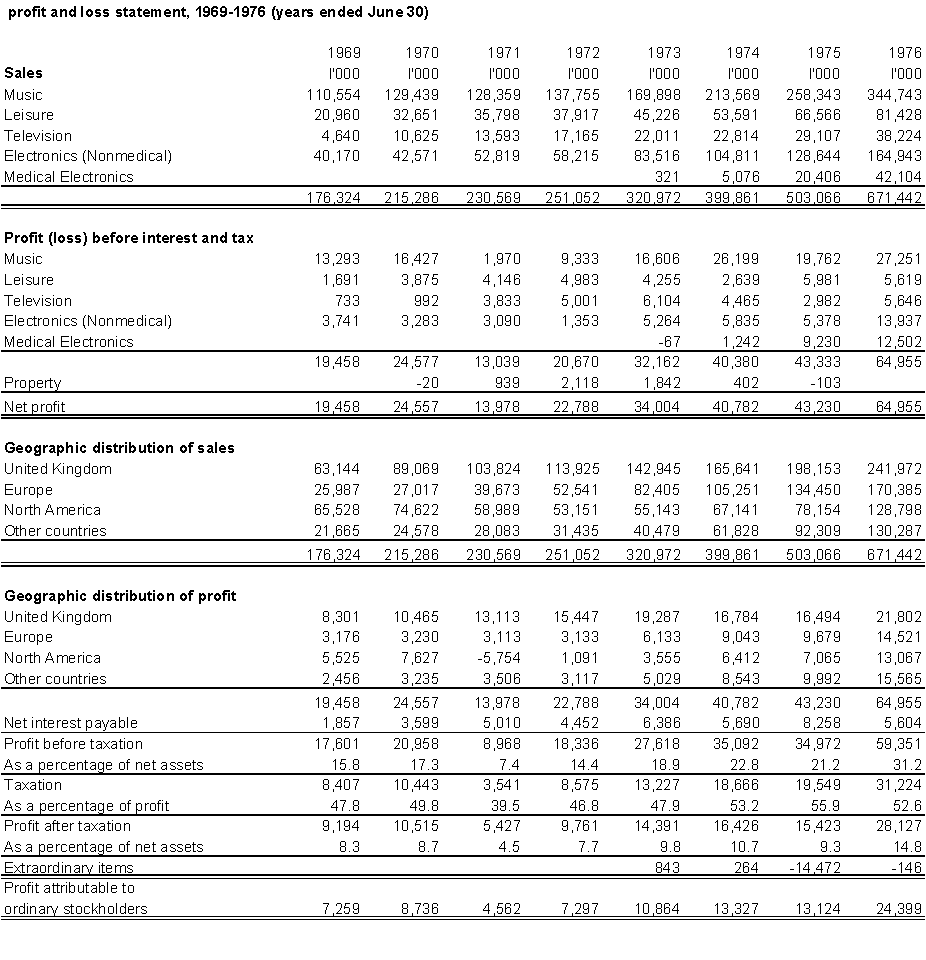
For EMI Ltd., it should have been the best of times. By 1977, six years after it introduced the world's first commercial x ray computed tomographic (CT) scanner, EMI's medical electronics operation had become wildly successful. In 1972, when the company placed the first CT scanner in a London hospital, EMI was a record production company with a small electronic components design and manufacturing division in Britain. In 1976, medical electronics contributed almost 20% to corporate pre-tax income. By 1977, computed tomography was an integral part of the company and EMI was expanding its now global medical equipment operation into nuclear medical imaging, ultrasonic imaging, and radiation therapy.
By the end of 1977, radiologists throughout the world had enthusiastically adopted the new instruments. EMI had sold more than 300 of the $300,000 to $600,000 CT units. Its manufacturing facilities in Britain and the United States were working beyond capacity. EMI quickly became a player in the global medical equipment industry. Upon entering the U.S. market in 1973, the company set up an American medical equipment design, manufacturing, and distribution system. Entering the Asia Pacific market in 1975, the company single-handedly raised British exports to Japan. Moreover, it was rumoured that the secretive Nobel Prize committee was considering honouring EMI engineer Godfrey Hounsfield with the coveted award.
Unlike most first-entrants, EMI adjusted to rapid technical improvements introduced by competitors. Although EMI lost the American market share lead in 1976, it regained the lead in 1977 by defeating several minor entrants and two major challengers. The decision to introduce CT scanners was a good one.
But it was the worst of times. EMI was about to lose its lead in the U.S. market again. Its early hold on the Japanese market no longer existed. And, as even stronger challengers appeared, EMI's tomographic R&D projects in Britain and the U.S. were fighting about design direction and competing for resources. What was going wrong?
This case addresses strategic issues in a rapidly evolving industry. The central theme of the case is that of a technological trajectory of product design, manufacture, and use. The case identifies points through which a new technological trajectory will commonly pass, competitive responses to movements along the trajectory, and strategic efforts to influence trajectory direction.
Business definition
Core product
X-ray computed tomographic scanners are one class of diagnostic imaging instrument, which are devices used by physicians and other health care workers to obtain information about physiological structures within the body. Several other methods of producing images preceded CT scanners, including conventional x-ray, electrodiagnostic, nuclear medical, and ultrasonic imaging equipment. Numerous other methods have emerged since the introduction of CT.
Conventional x-ray equipment became commercially available in 1896. Electrodiagnostic devices, such as electrocardiographs and electroencephalographs, were introduced during the early part of the century. Commercial prototypes of nuclear medical equipment were introduced during the early 1950s and achieved clinical acceptance in the 1960s. Similarly, ultrasonic imaging commercial prototypes became available in the mid 1950s, with clinical acceptance occurring during the late 1960s and early 1970s. Since the computed tomography commercialization of the early 1970s, nuclear magnetic resonance imaging, digital radiography, and several other imaging methods have been introduced as commercial prototypes; some have achieved clinical acceptance.
For some uses, CT represented a major advance over the conventional x-ray and other imaging techniques available in the early 1970s. Unlike conventional x-ray images, which superimpose organs and bones, CT creates an image of a single plane. By doing so, it ignores obstacles in front of the structure that the clinician wants to study. At the same time, by using a computer to interpret the transmitted rays, a CT scanner can reproduce finely detailed images. A conventional x-ray instrument produces images with only three to five shades of gray, corresponding to bone, fat, and muscle. Conversely, a CT instrument may produce an image with 300 or more shades of gray, often providing far more diagnostic information.
EMI's first instrument was a head-only scanner, with a price of about $300,000. Within two years, however, other companies introduced whole-body scanners, priced at about $500,000. EMI quickly responded with a whole-body instrument of its own. Although manufacturers' list prices differed, actual sale prices varied as manufacturers negotiated with buyers. For example, in order to obtain a placement in a high-profile research institution, a manufacturer might reduce its price significantly.
Users in the 1970s
The medical equipment market has several distinct segments. These segments include research hospitals, community hospitals, and private physicians offices. Medical CT followed the typical medical equipment adoption path through the segments. The path is found, with local variations, in medical equipment markets throughout Western Europe, North America, and Japan. The first users were physicians within research hospitals. Community hospitals began purchasing the instruments after determining that the instruments had clinical value. The community hospitals' decisions were influenced by the purchasing decisions at research institutions and the feedback that they received from doctors who were trained at teaching institutions. Private physicians, meanwhile, usually did not purchase the expensive systems.
Within the research and community hospitals, there is further market segmentation into medical departments. Each medical specialty, such as radiology or cardiology, has distinct needs, norms, and demands. Specialties often jealously guard their right to use particular equipment, fearing both incorrect diagnosis by less-practiced physicians and loss of referral income. In addition, departmental physicians and hospital administrators maintain an uneasy balance of power, with physicians having a major influence of the purchase decisions that are made, on paper at least, by administrators.
Traditionally, radiologists were the primary users of x-ray equipment, with some usage within the cardiology and neurology departments. The first physicians to find clinical use for CT instruments, however, were neurosurgeons. Where conventional x-ray, nuclear medical, and ultrasonic instruments had produced only dim indications of structure, CT produced clear images of lesions in the brain. In fact, CT's first competitive challenge was to nuclear medical imaging, where the primary market was for brain imaging. Unlike the nuclear medical brain imaging techniques then available, CT presented a structural image rather than a poorly resolved depiction of physiological activity. Subsequently, radiologists quickly adopted CT as their own, claiming the right to produce and interpret images for the neurosurgeons and other physicians. Although the instruments were used to produce images of structures within the body trunk, such as the liver and spleen, brain imaging remained the principal application.
The first CT instrument was placed in a British hospital in 1972. The scanners then spread slowly throughout Western Europe, being placed at a few research institutions in each country. By the end of 1977, about 200 CT instruments were being used in the Western European countries. The majority of the placements were in Britain, France, Germany, and Italy.
In the U.S., where the Mayo Clinic and Massachusetts General Hospital received the first CT scanners in 1973, the diffusion was much more rapid. Unlike Europe, where most of its hospitals were part of public health care systems faced with tight constraints on capital expenditures, the United States had a public health care payment system which provided indirect subsidies for medical equipment purchase. Although the federal Medicare and state-based Medicaid systems covered only a small proportion of the population, hospitals often built capital costs into the charges paid for by the public payment plans. Because private and not-for-profit insurers tended to follow Medicare's lead in payment decisions, they too provided indirect capital subsidies. In addition, federal hospital construction financing and equipment purchases by the federal Veteran's Administration Hospital system provided direct payment for medical equipment. American hospitals, therefore, had few constraints on their ability to pay for the expensive CT scanners. As such, by the end of 1977, approximately 1000 systems had been placed.
Japan, similar to most European countries, also had a broadly-based public health care payment system. CT diffusion occurred much more quickly than in Europe, however, because the Japanese Ministry of Health decided to support purchases on the equipment. After introduction of the first EMI CT system in 1975, placements grew at a rate similar to that in the United States. By the end of 1977, about 300 instruments were in use in Japan. Unlike the U.S., the Japanese system would not pay for the most expensive equipment. Thus, most instruments sold in the Japanese market were head-only scanners at the low end of the price range.
DistributioN AND SERVICE
New medical equipment is often complex, both in design and use. Because of the complexity, users and manufacturers rarely succeed the first time. Users must be trained and designs must often be readjusted based on user experience. The training and redesign information often passes through the sales and service system connecting the manufacturer and user. Although mature medical instruments may be sold via third-party distributors and sales representatives, advanced equipment in Europe, North America, and Japan usually must be sold and serviced by direct representatives of the manufacturer. Because of market segmentation within hospitals, it usually is necessary for a strong player in a medical equipment industry to have a distribution system dedicated to each medical specialty for which it produced advanced equipment. At the same time, a firm may benefit from economies of scope, by sharing distribution and service systems and leveraging reputation across different types of equipment.
User demands not only vary within medical specialties, but also across countries. Radiologists in Japan, the U.S., Britain, and Germany, for instance, may require different features on a core CT instrument. Buying patterns, too, may vary. Whereas central purchasing by multi-hospital systems is the norm in some countries (Germany, for instance), diffuse purchasing by individual hospitals is the standard in others (Japan, for one). Thus, to participate in a national market, a manufacturer must have access to a dedicated medical equipment distribution system within that nation.
Manufacturers must be able to provide after-sales service for complex medical equipment, in order to minimize down-time of the expensive instruments. Although independent service dealers exist, they usually do not have enough information about the equipment to do an adequate servicing job in the early stages of a new technological trajectory. At the same time, service contracts are a profitable source of revenue for manufacturers, often exceeding profits from system sales.
EMI moved quickly to set up direct CT distribution and service systems in Britain. By the end of 1974, the company also had a five-person direct sales and service operation in the United States, with an annual cost of about $250,000. In continental Europe and Japan, however, the company did not establish its own distribution and service systems. Instead, it relied on conventional x-ray companies to handle the scanner.
COMPONENTS
The principal components of a CT imaging system include an x-ray source, a detector, and a data processor. Other necessary components include monitors, film, and miscellaneous electronic parts. Because of its background in electronics manufacture, EMI was able to produce x-ray tubes and system consoles for its CT systems within corporate divisions. Other components were available from outside suppliers. Some could be acquired from suppliers to the conventional x-ray equipment manufacturers, others from scientific instruments manufacturers. Supplies of a few critical components lagged, however, causing manufacturing delays.
MANUFACTURING
Manufacturing issues in the industry include economies of scale and scope, complexity, and control of know-how. Some components enjoy economies of scale and scope across uses other than medical CT. Monitors, for instance, may be used in both CT systems and televisions. Data processing units may be used to record financial transactions as well as calculate high-resolution CT images.
Many components required in a CT system are complex, requiring tight manufacturing tolerances. The knowledge required to produce the components often is held by outside suppliers, rather than by CT manufacturers. As such, it is difficult for a new entrant to manufacture components with the necessary quality and cost. But just as individual manufacturing tolerances often are tight, so, too, are the interactions between manufacturing tolerances of several components. If the fit between two components is not good, the system will not work properly. The manufactured quality of a detector, for example, may affect the fit of an x-ray source.
EMI quickly set up CT assembly plants in Britain. However, the manufacturing back-log soon exceeded a year. Shipments throughout the world were far behind schedule. To supply the critical U.S. market, the company set up a Chicago assembly plant, which became operational in late 1976. For Japanese sales, the company decided not to set up a local manufacturing plant. Instead, EMI licensed its manufacturing rights to its distributor, Toshiba.
RESEARCH AND DEVELOPMENT
New medical instruments often first see life as research prototypes in academic institutions, usually paid for with public funds. Once the instruments reach commercial development stages, however, corporate funds and R&D capabilities are needed to refine them into clinically-useful form. To maintain long-run success, medical equipment manufacturers must possess technical and financial reserves. Because the direction of technical change usually is possible to predict, however, companies almost never possess all necessary development capabilities in-house. Therefore, they must be able to combine their internal know-how with key knowledge acquired from external sources.
The earliest x-ray computed tomographic devices were developed in American academic institutions. EMI then developed a research prototype in its corporate lab. The company's engineers fitted conventional x-ray and nuclear medical equipment components with data processors, and then used a simple algorithm to interpret and reconstruct the detected rays. But the basic design, the algorithm, and many of the components, soon proved to be inadequate. In keeping with the norm, EMI did not possess all the R&D capability needed to create a clinically-useful commercial instrument. It had to build its internal operation and acquire external know-how.
EMI quickly expanded the CT R&D operation at its Central Research Laboratory near London. In 1975, it also started hiring American engineers and set up an R&D centre at its U.S. plant. In both labs, the engineers combined internal developments with know-how, such as more advanced algorithms, developed by academic researchers. By 1977, the company was spending about $10 million a year on medical equipment R&D.
PROTECTING THE VALUE OF KEY RESOURCES
Methods of protecting the value of key resources include patents, copyrights, trade secrets, inimitable design and manufacturing know-how, and specialized supporting assets. Patents have sometimes provided entry barriers in the medical equipment sector. More often, though, they simply create a bargaining position after competitors have entered. CT followed the latter course. Although EMI filed for international patent protection, most competitors ignored the patents, hoping that they would be ruled invalid or that they could reach out of court settlements. EMI, in fact, had itself settled with an American academic researcher who had filed for a CT patent during the early 1960s. During the late 1970s, EMI filed suit against several of the American and European firms that introduced CT equipment.
Often more useful than patents on equipment are copyrights on service manuals and trade secrets surrounding service techniques. Once a company has built a user base for its equipment, if it can retain the service revenue it will enjoy a steady income. Members of a manufacturer's service staff frequently leave, however, to spawn new service companies.
Possibly the greatest protection of key resources is granted by possession of a broad set of supporting resources. A CT manufacturer that possessed in-house R&D capabilities, ties to external academic researchers, in-house manufacturing capacity, detailed distribution and service systems dedicated to a set of users, and established reputations with those users would be in an enviable competitive position. Although each of these supporting resources could be replicated over time, the replication often is slow.
CT TECHNICAL TRAJECTORY: FOUR GENERATIONS IN FIVE YEARS
The first commercial prototype of a new type of instrument usually has major problems. Often, improvements soon appear, usually introduced by competitors of the first entrant. Some improvements are minor. Others promise major quality differences. Although the distinction between a minor and major change frequently is unclear, it is common to identify some changes as the beginnings of new generations.
EMI's first commercial prototype was unusually successful. Nonetheless, by 1976 CT design had gone through four generations, with each new generation being introduced by a new competitor in the CT market. EMI's response to the generational changes was critically important to its performance.
The generational changes drastically improved image quality. CT image quality is affected by both scanning speed and detector method. By increasing scanning speed, motion artifacts created if the subject moves while being scanned are reduced. By reducing boundaries between detectors, image artifacts created by inadequate detection of x rays that have passed through the body can be reduced. Generations one through four of CT commercial instrumentation were defined by scanning and detection methods.
FIRST GENERATION - 1972: EMI's first research prototype, constructed in 1967, required nine days to obtain the data and two and a half hours to compute the image. By 1972, when it introduced its first generation commercial prototype head scanner, scanning speed had been reduced to five minutes. The first-generation system produced its image by using a fixed detector to capture a single beam of rays that moved around the head in small incremental steps.
Variants of the first-generation design were quickly introduced by competitors. In 1974, two firms entered the CT market. Neuroscan, a California startup, introduced a head scanner and Disco, a small Maryland firm, introduced a whole-body scanner designed by a Georgetown University researcher. In 1975 the Georgetown whole-body design was acquired by Pfizer, a large American drug company with no prior experience in the diagnostic imaging industry. EMI's British engineers quickly designed a body scanner and the company responded successfully to these challenges.
SECOND GENERATION - 1975: Second generation systems were introduced in 1975. These systems used two incrementally-advancing beams, reducing scan times to less than two minutes. They also incorporated improved reconstruction algorithms. The first to appear was a head scanner manufactured by Syntex, a California drug company, under license from Stanford University. The next was a body scanner designed by Technicare, a leading nuclear medical imaging manufacturer. The Syntex system had little success. The Technicare whole-body scanner, however, was an immediate hit. In 1976, the company was the market leader in systems placed in the U.S. Technicare also made quick in-roads into the European and Japanese markets, distributing its scanners through the long-established x-ray equipment manufacturers Siemens, which earlier had distributed Technicare's innovative nuclear medical equipment.
At first, EMI was able to respond to the Technicare challenge in the U.S. market. In 1976, it introduced its own second-generation body scanner, designed in its British research lab. At the same time, EMI expanded its American sales and service system. Technicare, meanwhile, was having manufacturing problems. The nuclear medical company also was finding it difficult to break into the established market of conventional x-ray equipment users, where most of CT sales were now taking place. EMI regained the American lead in 1977. During 1978, however, Technicare surged ahead once again.
THIRD GENERATION - 1975: Third-generation systems also appeared in 1975. Rather than one or two pencil beams of rays, these systems used a fan beam generated by a rotating x-ray source. The broader beam reduced scan times to about 5 seconds. A key advance underlying these systems was the development of new algorithms by academic researchers.
The first third-generation system was a head scanner introduced by Artronix, a minor nuclear imaging computer system manufacturer. The Artronix system had little impact. But by the end of 1977, third generation body scanners had been introduced by General Electric, Varian, and Searle. GE, an established x-ray equipment manufacturer, had combined in-house research with experience gained by briefly manufacturing the Neuroscan first-generation system. Varian, a California electronics equipment manufacturer with experience in computing and x-ray tube production, licensed its core third-generation design from Stanford University. Searle, a large pharmaceutical firm and a leader in the nuclear medical imaging, hired engineers from a leading x-ray equipment manufacturer to complement its in-house computing staff. Varian and Searle had little success, and left the CT market during 1978. GE, however, was much stronger, matching EMI's placements during 1978 and threatening Technicare's position as market leader.
General Electric was the first of the long-established x-ray equipment leaders to make a strong move into the CT market. GE begun an in-house first-generation technology research project in 1974, designing a mammography research prototype that it chose not to introduce commercially. Instead, GE licensed the Neuroscan first generation head scanning system in 1975 and then begun work on the third-generation system. With the exit of Varian in 1977, GE augmented its third-generation work by acquiring rights to Stanford University third-generation CT patents that had been held by the California company.
Several other x-ray equipment leaders were waiting in the wings. Siemens began a first-generation research project in 1974, entered the European market about 1975, and in late 1977 was ready to introduce a third-generation system to the U.S. market. Philips began a second-generation research project in 1975 and introduced it to American and European markets in 1977. Picker funded CT research at Washington University in St. Louis, beginning in 1975, and by 1977 had constructed an in-house research prototype. Compagnie Generale Radiologie (CGR), an established French radiology firm, was working on a CT design in France while distributing the Pfizer and Varian systems in Europe.
Technicare and EMI had to decide how to respond to GE's third-generation challenge and to the entry of the other imaging industry leaders. Technicare rushed its own third generation system to market in 1978 but, because it suffered technical flaws, the system was received poorly. EMI, meanwhile, was not sure how to respond. Its British operation was convinced that it could improve the second-generation system, which could be priced significantly below the GE fan-beam scanner. Accustomed to the cost-constrained British market, the London operation expected the price-performance trade-off to be acceptable. EMI's American operation, was convinced that the American market would both demand faster scan times and be able to pay for the more expensive equipment. In 1978, therefore, EMI's American operation acquired Searle's third-generation design and engineers when Searle left the CT market. While the British engineers worked on second-generation improvements, EMI's American employees worked on the third.
FOURTH GENERATION - 1976: To further muddy the waters, a fourth-generation system appeared. Third-generation CT scanners used a rotating source and rotating detector, with the detector rotation creating motion artifacts in the images. During 1976, American Science & Engineering, an industrial x-ray company, introduced a system that used a rotating source and fixed detector. The system, which had been built under a contract with the National Cancer Institute, promised a significantly improved image quality.
American Science & Engineering had little success with its fourth-generation scanner. It licensed the scanner to Pfizer in 1978. Other firms, however, began to adopt the design. In 1977, Picker, a long-established diagnostic imaging industry leader, introduced a fourth generation system. During February 1977, EMI, too, began a fourth-generation development project in its U.S. operation.
CROSS-CURRENTS OF INDUSTRIAL FORTUNE
In addition to the rapid changes in the design of the core product, EMI and other members of the CT technical subfield of the diagnostic imaging industry were subject to changes in several key elements of the industrial environment.
SOCIAL CROSS-STREAM
Several of the influences on CT manufacturers emanated from the social environments, which strongly affected diffusion of the equipment. CT diffused slowly through Europe, constrained by public limits on payment. In the U.S., though, the instruments were wildly successful. Every medical institution in the country wanted one, in order to improve care and compete with other hospitals. Almost every hospital expected to be able to pay for the equipment through indirect use of federal, state, and private medical insurance payments.
By the end of 1977, the third-party payers in the American insurance system were showing signs of being unwilling to bear the cost of expensive equipment. Most states were establishing Certificate of Need programs, which would require regulatory approval of equipment purchases over some limit, usually about $150,000. Although some manufacturers responded by selling their CT systems in modules, with each module priced at $149,000, the industry feared that orders would drop.
At the same time, a few large states were experimenting with prospective payments systems for medical insurance payments. Under the Medicare and Medicaid programs that were established during the late 1960s, hospitals billed public insurers on the basis of their actual costs. The new prospective payment systems, however, would pay hospitals a fixed amount for each patient admitted, with the payment based on the class of illness. It seemed likely that hospitals would find it harder to build capital costs into operating charges.
USERS
Changes, and potential changes, in user demands also were on the horizon in late 1977. Demand growth for CT imaging equipment during the mid-1970s was phenomenal. After first finding a niche in neurosurgery, the equipment then was adopted by radiologists. As physicians throughout the world rushed to adopt the technology, almost any entrant to the industry was assured of at least a few sales. However, in most medical institutions, radiologists had long-established relations with the major x-ray equipment companies. Accordingly, a hospital might be GE shop or an entire region might buy a large proportion of its equipment from Picker.
By the end of 1977, two key changes were underway. The first change, felt in markets throughout the world, was that users were less willing to buy equipment from new entrants to the industry. With the quick exit of several early innovators, many early adopters were left with expensive equipment that did not work well and could not be fixed. Manufacturer stability had become a key issue.
The second change was felt mainly in the U.S. As a response to state-based regulatory limits on capital expenditure, many physicians and hospitals set up out-patient centers, which were not subject to the regulations, to purchase CT equipment. A related change was the growth of a mobile equipment market. Several mobile imaging equipment companies were set up by medical entrepreneurs, who usually bought scanners from CT manufacturers and then installed them in trucks to create mobile clinics. One company, Omnimedical, even acquired the assets of the bankrupt first-generation CT scanner manufacturer Neuroscan, licensed second-generation designs from EMI, and then began to manufacture its own scanner. With restrictions on capital expenditures, many hospitals were willing to share the use of CT scanners. But whether the new centres and mobile companies would exhibit the same loyalty as hospitals to established firms was unknown.
Two other factors in the user environment threatened to reduce demand. One factor was technical confusion. Neither manufacturers nor users could predict which of the third and fourth generation systems would succeed, or whether a refined second generation system might prevail. Rather than risk buying a product that would not be supported with ongoing service and refinement, many potential buyers were sitting back, waiting for the technical uncertainty to subside.
Another factor was a pipeline effect. By late 1977, many early adopters already possessed CT instruments. Rather than buy second instruments, many research physicians were now working with the CT system they already possessed to find out what clinical value the instruments might offer.
PRODUCT CROSS-STREAM
When commercial CT was introduced to the world in 1972, the imaging industry consisted of the conventional x-ray, electrodiagnostic, nuclear medical, and ultrasonic imaging subfields. Computed tomography equipment quickly become an important part of the diagnostic imaging industry. Although the x-ray product segment remained the largest, CT cut into sales of conventional x-ray equipment. At the same time, it drastically reduced sales of nuclear medical imaging equipment, which had found its largest application in the neurology market segment that had been the first to adopt CT instruments. Only ultrasonic and electromedical equipment remained largely untouched by the CT innovations.
Conventional x-ray and nuclear medical researchers and manufacturers responded to the introduction of CT equipment in two ways. The leading x-ray and nuclear medical instrument manufacturers often introduced CT equipment. Many academic researchers, however, responded by incorporating some of the computed tomography ideas into their own research. In nuclear medicine, emission computed tomographic research, which had been underway since the early 1960s, became much more popular. Meanwhile, in radiography, researchers stepped up programs in electron and digital radiography. By the mid-1970s, several manufacturers were developing academic and in-house nuclear emission computed tomographic systems and new radiographic systems.
In addition to improvements to the existing equipment, several new types of imaging equipment were on the horizon. Thermographic instruments, which obtained images by heating the body, recently had been introduced commercially. Nuclear magnetic resonance imaging (NMR or MRI) equipment, which would obtain images by measuring changes in distribution of atomic nuclei within the body, were being designed at academic institutions in the United Kingdom, the U.S., and Japan. By the end of 1977, a few corporate manufacturers, including some imaging industry incumbents, had also started NMR research programs.
Imaging equipment markets throughout the world were seeing the expansion of global manufacturers during the 1970s. Although the markets remained national, since few buyers crossed national boundaries to purchase equipment, the industry did not. In the U.S., which comprised about 35%-40% of the global market for medical equipment, many international manufacturers were now offering imaging equipment. During the 1950s and early 1960s, the U.S. market had been served primarily by American manufacturers, with the European firms Philips and Siemens also playing important roles. By the late 1970s, however, many European firms with experience in their home markets had entered the U.S. Most specialized in one technical subfield of the industry. A few were offering a broad line of product types.
At the same time, several Japanese manufacturers were beginning to expand in the European and U.S. markets. Japanese companies had sold components and private label systems since the 1950s and 1960s. A few firms had then introduced their own brands, usually sold by domestic distributors, during the 1960s and 1970s. Starting with Toshiba in 1975, major Japanese firm were now beginning to set up direct distribution systems in the United States and Europe, usually selling low-cost, relatively simple imaging equipment. Most Japanese manufacturers, whether selling under their own name or not, specialized in products from one diagnostic imaging technical subfield, such as electrocardiographic or ultrasonic equipment. A few, however, were providing instruments from most or all subfields.
EXISTING MANUFACTURERS
During the early 1950s, there were perhaps 100-150 imaging equipment manufacturers in the world, with about 40 selling equipment in the United States. Most developed countries had one or more strong national x-ray and electromedical equipment manufacturers, with a few of those firms serving international markets. In the U.S., the leaders were General Electric, Picker X-Ray, and Westinghouse. Several small specialists, including Continental X-Ray, Profexray, and Standard X-Ray held significant positions. The European firms Philips and Siemens had strong positions in their home markets and smaller, but notable, positions in the United States. Other national leaders included CGR in France; Hitachi, Shimadzu, and Tokyo Shibaura in Japan; and GEC in the United Kingdom.
During the 1960s and 1970s, the number of imaging equipment manufacturers grew explosively. By the mid-1970s, the U.S. market alone was being served by more than 100 manufacturers. In the U.S., however, the leaders remained the same, with General Electric, Picker, Philips, Siemens, and the Westinghouse system controlling about 70% of the American non-CT imaging market in 1977.
Although the leaders had maintained their positions in the market, two of the leading players had gone through changes in corporate ownership during the growth period. Picker, an imaging equipment specialist, was acquired by a financial services company, CIT Financial, in 1958. Although members of the Picker family continued to manage the medical equipment subsidiary, the senior members were reaching retirement age during the mid-1970s. The Westinghouse x-ray operations were acquired by the experienced French radiology equipment firm, CGR, in 1971. Subsequently, CGR was acquired by the diversified French company, Thomson, in 1975.
A few new players also had some success in the United States. Litton had a strong x-ray position, built on its acquisition of Profexray in 1964. The drug company, G.D. Searle, had become an important player in nuclear medical imaging via its acquisition of the nuclear imaging innovator Nuclear Chicago in 1966. A financial services firm, Boston Capital Corporation had turned into a nuclear and ultrasonic imaging equipment manufacturing specialist, Technicare, by acquiring two innovative startup companies during the early 1970s. EMI, meanwhile, had built on its CT success to enter the nuclear and ultrasonic imaging fields.
In addition to firms in the broader industry, EMI also faced competition from CT equipment specialists. Several firms without imaging system experience had seen CT as their entry point into the industry, with Pfizer, Syntex, and Varian being among the most notable. Although based in the United States, the three firms also sold equipment in Europe and Japan, using local medical equipment manufacturers and distributors.
Several companies were weak in the American and international markets during the late 1970s, but had strong non-American domestic presence. Several Japanese firms are particularly notable. With decades of experience in manufacturing conventional x-ray equipment and several important x-ray and ultrasonic innovations to their credit, these companies were potentially strong competitors in global markets.
GENERAL ELECTRIC: General Electric was among the first to introduce x-ray equipment, entering the market within a year of Roentgen's 1895 discovery of the rays. GE was a leader in American conventional x-ray equipment sales during 1977, with the largest radiology direct distribution system in the U.S., and held a minor position in the electrodiagnostic subfield. GE held a significant but much smaller share of European markets and had a minor presence in Japan. The large, diversified, and profitable electrical and electronics equipment manufacturer had long had a reputation for being a slow-moving but powerful player in the imaging field. Although GE had distributed nuclear imaging equipment since the early 1970s, the company had only recently undertaken manufacturing entry, by acquiring an innovative but financially weak nuclear company. It had not yet begun to produce ultrasonic imaging equipment, although it was sponsoring academic ultrasonic research.
PICKER: Picker began producing x-ray equipment in the early part of the century. The imaging specialist was acquired by CIT Financial during 1958, but was still run by members of the Picker family during the late 1970s. Picker was an important innovator in the nuclear and ultrasonic imaging fields during the 1960s, being the first of the major American x-ray equipment producers to enter the new product areas.
SIEMENS: The German firm Siemens was a large, profitable, and broadly diversified industrial products manufacturer. Siemens introduced commercial x-ray products during 1896. The company had long held a major position in European markets x-ray equipment markets and minor shares in the U.S. and Japan. During the late 1960s, it drastically expanded its United States operation and by 1977 was one of the strongest players in the U.S. conventional x-ray market. It also held minor shares of international ultrasonic and nuclear imaging markets, built on a combination of in-house development and distribution of innovative small firms' products.
PHILIPS: The Dutch firm Philips was another long-time leader in international x-ray equipment markets. With experience in industrial and consumer electronics, the company had a strong financial and technical base. Its U.S. and European operations had been separated during World War Two, however, and often had problems coordinating product and market development. The company was slow to move into emerging subfields of the imaging industry. It held a tiny non-U.S. position in nuclear imaging and had just acquired, in 1976, an innovative American ultrasound firm. Philips, though, was perhaps the first manufacturer to start a nuclear magnetic resonance imaging research program, as its industrial spectroscopy division had very early seen the technical and commercial potential of academic magnetic resonance medical research.
THOMSON/CGR: Partly supported by government-supported preferential buying, CGR had long been successful in France, where it had operated since before World War II. However, it had not been able to acquire a significant market share beyond the French borders. Its acquisition of the Westinghouse x-ray operations in 1971 was intended to give it strength in the U.S., but that strength had not yet developed. By 1977, CGR had not yet entered the nuclear or ultrasonic imaging subfields of the industry, although it was rumoured to be using the resources of its new parent, Thomson, to finance development programs for products in those fields.
G.D. SEARLE: Searle, a leader in the pharmaceutical industry, entered the imaging industry in 1966 by acquiring a small innovator, Nuclear Chicago. The marriage between the equipment research-oriented personnel in the Nuclear Chicago operation and the shorter-horizon drug company had not always been smooth, and Searle had carried out a major reorganization of its imaging operation during the mid 1970s. But the company remained a nuclear market leader and product innovator in 1977, financing a large emission computed tomography research program. It also had just introduced an innovative ultrasonic scanner. The imaging operation was under pressure, however, as the key nuclear patents to which it held rights were about to expire and the ultrasonic scanner was not being well-received. Searle had not entered the conventional x-ray subfield of the industry.
LITTON: Litton was a strong aerospace firm that entered the imaging industry as part of a diversification spree during the 1960s. It attained a secondary leadership position in the conventional x-ray field and used its distribution system to handle Toshiba's ultrasound and nuclear lines. But Litton's attempt to introduce its own nuclear and ultrasonic imaging devices had been unsuccessful in the market, although one of the company's ultrasonic technical advances had been quickly adopted by its competitors. During 1977 it was carrying out active negotiations with an x-ray innovator, Xonics, with the goal of divesting its imaging lines.
TECHNICARE: Similar to Searle, Technicare was a non-x-ray imaging specialist. The firm was profitable, but its financial base was smaller than those of its large competitors. Once a financial services firm, it had acquired a nuclear imaging innovator in 1971. Continuing to innovate, it had quickly become a major player in that subfield. In 1975, Technicare then acquired an ultrasound equipment innovator and had some success in that field. The company had strong but narrow in-house electronics capabilities, primarily focused on a few key people. The company had entered the CT market by drawing on its knowledge of nuclear imaging data processing. Its second generation design, which it had brought from idea to commercial prototype in less than a year, was a quick success. By early 1978, though, the system was under pressure from the third and fourth generation products being introduced by other entrants and Technicare was rushing a third-generation system of its own through research and development stages.
PFIZER: Similar to Searle, Pfizer had a strong financial base in its pharmaceutical operations but lacked medical equipment technical expertise. Where Searle had built an in-house medical equipment technical operation, based on its Nuclear Chicago acquisition, Pfizer pursued an external technology development strategy. To enter the CT subfield, in particular, it had licensed a design from Georgetown University. Pfizer also was sponsoring NMR research at the University of California in San Francisco and, in 1976, purchased a small specialist x-ray equipment company. The company had enjoyed one year of early CT success in the U.S., Europe, and Japan, but its first-generation body scanner quickly was surpassed by other firms and it had not been able to respond. The company's small direct sales operation in the U.S. and European and Japanese distributors were having little success by 1977 and Pfizer was shopping for a new design.
SYNTEX: Syntex was yet another drug company that saw CT as a logical extension of its business. Licensing designs from Stanford University, the firm introduced an early second-generation product, but met little success and quickly withdrew.
VARIAN: Varian is a California electronics equipment manufacturer. Long a producer of x-ray tubes, the company saw innovative imaging equipment as an entry point into the systems market. After acquiring rights to new Stanford University research and the patents previously held by Syntex, Varian introduced an early third-generation system. About the same time, the company also began a technically advanced ultrasonic scanner development project. By 1977, however, it was clear that the Varian CT system had technical problems and that the company had no visibility in the user market.
HITACHI: Hitachi Medical Systems was a part of a financially-secure, broad-based electronics equipment manufacturer. The company had a major position in Japanese x-ray and ultrasonic markets and minor positions in international markets. In 1977, it introduced a CT scanner to the Japanese market, but did not begin to sell it on international markets.
TOSHIBA: Tokyo Shibaura, which adopted the name Toshiba during the early 1970s, began manufacturing x-ray equipment early in the century. A broad-based industrial and consumer electronics firm, the company had financial and technical strength. A major player in the Japanese market, Toshiba also sold x-ray components and private-label systems in the U.S. During the 1970s, it introduced ultrasonic and nuclear imaging equipment to the U.S. and Europe, selling through distributors. In 1975, it set up a small direct American distribution system, concentrating on inexpensive and relatively simple products. Toshiba was distributing EMI's CT scanner in Japan and had its own system under development by early 1978, building on a design that it licensed from the British firm.
SHIMADZU: Shimadzu was another financially secure, broad-based electronics manufacturer. The company had manufactured x-ray equipment since 1911 and held minor international positions to complement its strong place in the Japanese market. During late 1977, it was rumoured to be developing a CT scanner.
JEOL: Jeol was another Japanese electronics equipment manufacturer. The company was among the earliest corporate manufacturers to undertake magnetic resonance imaging equipment research. Similar to Philips, it drew on its experience with industrial spectroscopy. About 1976, it too had begun an in-house CT development program. However, no commercial product had emerged by the end of 1977.
EMI'S DILEMMA
EMI used its CT success to expand into other imaging industry subfields. In 1975, EMI acquired a Scottish firm, Nuclear Enterprises, that had been an early nuclear medical and ultrasonic imaging equipment innovator. During 1977 EMI also was sponsoring nuclear magnetic imaging research at Nottingham University and Hammersmith Hospital in London. By late 1977, though, the company faced a dilemma.
Although a broad-based electronics and music production company, EMI's corporate financial resources were being strained during the late 1970s. Part of the strain coming from its medical imaging expansion. Part, too, had occurred because of reverses in its music division.
EMI had been able to overcome the early challenges mounted by Pfizer and Technicare. In 1977, however, it faced a renewed challenge from Technicare and emerging challenges from several other firms. Could it build on its early success? Or was its loss of market share permanent? How should the company respond?
Exhibit 1 Four Technical Generations of CT Scanners
First Generation First prototype in 1967 requited nine days to obtain data and 2.5 hours to compute an image. First commercial prototype in 1972 scanned around the head in incremental steps, producing an image in five minutes. Body scanner introduced in 1975 by EMI, DISCO and Pfizer. Used a single beam of rays.
Second Generation Head scanner introduced in 1975 by Syntex with little success. Used two incrementally advancing beams. Reduced scan time to less than two minutes. Incorporated improved reconstruction algorithms. Technicare introduced whole body scanner in 1976. EMI introduced its second generation body scanner in 1976.
Third Generation Used a fan beam generated by a rotating x-ray source instead of one or two pencil beams. Reduced scan times to approximately five seconds. Utilized new algorithms. First introduced in 1975. EMI acquired Searle's design in 1978.
Fourth Generation Introduced in 1976 by American Science & Engineering. Used a rotating source and a fixed detector. Promised improved image quality. EMI started a fourth generation project in its U.S. labs in early 1977
Exhibit 2. Market shares (%) in the U.S. CT market, 1973-1977.
73 74 75 76 77
EMI 100 98 71 37 41
Neuroscan 2
1 1
DISCO 1
Pfizer 13 8 8
Technicare 11 46 20
Syntex 1 3 4
Artronix 1 1 3
General Electric 1 3 17
AS&E 1 1
Picker 1
Philips 1
Searle 1
Varian 2
Siemens 1
100 100 100 100
100
Exhibit 3. U.S. sales of CT devices and all diagnostic imaging systems, 1973-1977
CT All imaging
($ million) ($ million)
1973 7 450
1974 20 550
1975 80 650
1976 160
750
1977 360 1,000