Lipitor: At the Heart of Warner-Lambert
Introduction
November 1999 was a tense month in the Morris Plains, New Jersey headquarters of Warner-Lambert Company. On November 3, Warner-Lambert and American Home Products (AHP) announced an ambitious merger that would create the world’s largest pharmaceutical company, with over $26 billion in combined annual revenues. The following day, Pfizer, Inc. announced an unsolicited $80 billion stock offer for Warner-Lambert, the largest hostile takeover attempt in the history of the pharmaceutical business.
Late on November 29, Warner-Lambert President and CEO Lodewijk J.R. de Vink settled in to read Pfizer’s response to his latest move, a legal suit seeking to terminate the co-marketing agreement between Warner-Lambert and Pfizer for the wildly successful cholesterol-lowering drug, Lipitor. Pfizer’s short news release called Warner-Lambert’s lawsuit the latest in “a series of desperate defensive measures designed to deny its shareholders the opportunity to consider a superior offer from Pfizer.”[1] As de Vink reread the Pfizer statement and contemplated his next move, he recalled the chain of decisions that made Lipitor the engine of Warner-Lambert's recent success and the key prize in the ongoing merger battle.
Pharmaceutical Industry Overview
The pharmaceutical industry is a critical element of health care delivery around the world. In 1998, sales of ethical pharmaceuticals reached $103.5 billion in the U.S. alone.[2] The U.S. is the single largest purchaser of prescription drugs, representing approximately 36% of the worldwide market.[3] As illustrated by the fact that in 1998 the largest pharmaceutical company captured only 6.5% of U.S. pharmaceutical sales, the industry is fragmented (Exhibit 1).[4]
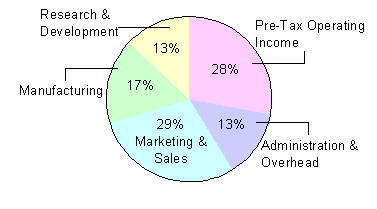
The economics of the pharmaceutical industry are different than in many other industries. Figure 1 breaks down the major expenditures of a drug company as a percentage of sales. Marketing and sales demands translate into the single largest expense incurred by the industry. Sales forces have nearly doubled in the past five years to more than 62,000 field representatives.[5] During the same period, federal regulations were eased, enabling drug companies to promote their products more like consumer goods. This has spurred the industry’s onslaught of direct to consumer (DTC) television and print advertisements. In total, DTC spending was an estimated $1.2 billion in 1998[6]. Drug manufacturing costs are relatively low at only 17% of sales, but research and development investments are a higher percentage of sales than in virtually any other industry, including the electronics, aerospace, and automobile industries.[7]
Figure 1: Drug Company
Functions as a Percent of Sales[8]
Pharmaceutical companies must
invest heavily in research and development (R&D) to bring a new drug to
market. R&D investment has steadily increased over the past two decades and
is expected to reach $24 billion in 1999.[9] The entire process of discovering and
developing a new drug is long and complex (Exhibit 2). It is estimated that only one out of nearly
10,000 chemically synthesized molecules investigated as drug candidates
actually becomes an approved drug.
During the 1990s, the average length of time required for drug
development and government approval reached 15 years in the U.S.[10] As the length time to bring a drug to market
has increased, so have the costs, economic risks and uncertainties. One study estimates the pre-tax cost of
developing a drug introduced in 1990 to be $500 million.[11] This approximation is inclusive of the cost
of research failures as well as successes.
Moreover, most pharmaceutical companies continue to invest in research
even after a drug has been introduced because additional clinical trials
enhance the therapeutic knowledge and commercial potential of currently
marketed drugs.
The costs of drug development are further intensified because of increased competition in the pharmaceutical marketplace, which has led to shorter exclusivity periods. The time during which the first drug in a therapeutic class is the sole drug on the market is shrinking. For example, Mevacor, the first drug in the groundbreaking cholesterol-lowering class of drugs called statins was launched in 1987, but the second compound in this drug class, Pravachol, was not on the market until 1992. Mevacor enjoyed an almost four-year exclusivity period. This is in contrast to Invirase, the first in a new class of AIDS drugs called protease inhibitors, which entered the market in November 1995, only to have two competitors, Norvir and Crixivan, approved less than four months later. The time that companies have to recoup development costs to fund future R&D efforts has been severely compressed. Drug companies were traditionally only concerned about the effective patent lives (time on the market before patent expiration) of their drugs, which have averaged between 11 and 12 years over the past decade.[12] But, patent life can no longer be used as a sole indicator of how long a product has to reap returns in the marketplace. Patent protection for drugs remains in effect, but comparable or superior drug substitutes are available quicker than they have ever been.
Another area of concern for pharmaceutical companies is the generic drug market. Generic drugs are low cost copies of products no longer patent protected. Once a drug loses patent exclusivity, other companies are free to petition the FDA for approval to manufacture and market generic substitutes. On average, generics are discounted 86% versus full price, branded drugs.[13] This discounted pricing is economically feasible because the generic manufacturers do not have significant development and marketing investments to recover. With cost containment pressures increasing in the health care arena, market share of generic drugs has more than doubled in the past decade; they accounted for 41% of all prescriptions written in 1998. [14],[15]
To combat the growing cost of R&D, shorter life cycles and cost-containment pressures, pharmaceutical companies are forming more strategic alliances. The total number of alliances grew from 121 in 1986 to 627 in 1998.[16] In addition to alliances, mergers and acquisitions have also become larger and more frequent since the mid-1980s.[17] Pharmaceutical industry experts often debate the merits of these types of relationships, but experience says that these relationships can allow pharmaceutical companies to leverage others’ research expertise, as well as bring products to market quicker and more effectively.
Warner-Lambert Company History
Warner-Lambert Company has evolved considerably throughout its history to become one of the world’s leading pharmaceutical companies. A Philadelphia pharmacist, William Warner laid the foundation of the current company in the early 1850s when he created a process to coat tablets of harsh tasting medications with a sugar shell. Over the years, the company expanded through many mergers and acquisitions to become an international competitor in several businesses. Today, Warner-Lambert is focused on three primary business segments: consumer health care products, confectioneries and prescription pharmaceuticals (Exhibit 3).
The consumer health care products business is comprised of over-the-counter (OTC) medications, shaving products and pet care products. The company owns the well-known Rolaids, Lubriderm, Benadryl, Listerine, Schick and Tetra brands. The confectionery business consists of a broad line of chewing gums, bubble gums, breath mints and cough drops including the like Trident, Chiclets, Certs and Halls brands.
The prescription pharmaceutical business segment is certainly the most substantial contributor to Warner-Lambert’s bottom line, not only representing 57% of all sales, but also having the highest profit margin (Exhibit 3). Warner-Lambert has always been involved in medicine, but it reinforced its commitment in 1970 through the acquisition of Parke-Davis, a company with a rich past in prescription pharmaceuticals. Parke-Davis was responsible for developing a system for standardization of medicines before the turn of the century and it also created the first organized, systematic method for clinical testing of new drugs. In the 1950s, Parke-Davis was considered the biggest drug company in the U.S.
Unfortunately, Parke-Davis did not maintain its pre-eminence under the Warner-Lambert umbrella. Warner-Lambert spent the early 1990s recovering from a series of misfortunes in its pharmaceutical business that earned the company bad press and poor ratings from industry analysts. Warner-Lambert’s confectionery and consumer health care businesses carried the pharmaceutical division. In 1993, Lopid, the company’s leading drug, lost patent protection and the planned successor did not receive Federal Drug Administration (FDA) approval, so the company was left with nothing in position to mitigate the revenue loss from Lopid. That same year, the FDA cited Warner-Lambert for lack of regulatory compliance at two Puerto Rico manufacturing facilities. Eight drugs were recalled and manufacturing came to a halt while the plants were brought into compliance. The early 1990s also saw proposals for sweeping changes in the U.S. health care system-changes that threatened pharmaceutical companies. The industry reacted by down-sizing work forces and shrinking R&D budgets. Warner-Lambert laid off employees throughout the organization, including employees at the Parke-Davis research facility in Ann Arbor, MI and reined in funding for R&D. [18] The situation was so dismal that industry analysts were questioning whether Warner-Lambert could survive as a serious pharmaceutical competitor. In May 1994, David Lippman of Prudential Securities concluded that the Warner-Lambert pharmaceutical business had “no growth potential,” and that the R&D pipeline was “not sufficient to put the company back in the prescription drug game”.[19]
Warner-Lambert has several therapeutic areas of interest for drug development including cardiovascular disease, central nervous system disease, diabetes, women’s health and anti-infectives/antivirals. Research in cardiovascular disease led to the discovery and development of a powerful cholesterol-lowering agent marketed under the brand name Lipitor (chemical name atorvastatin).
Coronary Artery Disease
Coronary artery disease is the leading cause of death in the U.S. Each year more than a million Americans suffer a heart attack, of which about half are fatal. A leading cause of coronary artery disease is the buildup of plaque in the blood vessels, which leads to blockage, heart attacks and strokes. Frequently, this plaque buildup results from excessive cholesterol, which is transported in the bloodstream from the liver by low-density lipoprotein (LDL) and returned to the liver for elimination by high-density lipoprotein (HDL). In addition, new scientific data shows that when levels of another type of cholesterol, triglycerides, are elevated, plaque build-up can also result. Cholesterol is produced naturally by the body, in addition to being ingested through many foods. Efforts to lower LDL-borne cholesterol and triglycerides and raise HDL-borne cholesterol can have major benefits in reducing the risk of heart disease and improving overall cardiovascular health. As many as 50 million Americans are thought to have excessive LDL cholesterol levels; however, only an estimated 6 to 8 million are treated.[20]
Role of Statins in Cornary Artery Disease Treatment
Patients with high LDL cholesterol were historically treated with medicines that broke down cholesterol regardless of whether it was naturally produced by the body or from ingested food. While somewhat effective, these medicines did not prevent more cholesterol from being produced by the body. In addition, they caused side effects such as stomach pain and nausea.
The discovery of statins, a new therapeutic drug class, revolutionized the treatment of high cholesterol. Drugs known as statins have a unique method of action: they inhibit a key enzyme in the body from producing cholesterol. Rather than waiting for cholesterol to be produced and then attempting to reduce the overall levels, statins directly intervene in the body’s production of cholesterol. As a result, this makes statins much more powerful than the older medications. In addition, statins are more convenient to use because they are dosed as tablets rather than liquids and cause few side effects, making them easier and safer for patients to take.
More than 45 years of research led to the discovery, development and the 1987 Merck launch of the first statin, Mevacor. On the coattails of Mevacor’s success, three more statins entered the marketplace. The market shares for the statins available just prior to the launch of Lipitor are listed in Figure 2.
Figure 2: U.S. Market Shares of
Cholesterol-Lowering Drugs, January 1997[21]
|
Drug
Name |
Manufacturer |
Launch Year |
Market Share |
|
Mevacor |
Merck |
1987 |
14% |
|
Pravachol |
Bristol-Myers Squibb |
1991 |
21% |
|
Zocor |
Merck |
1992 |
32% |
|
Lescol |
Novartis |
1994 |
14% |
Note:
Market shares are based on the entire cholesterol-lowering drug market (not
only statins)
Lipitor Research & Development
The challenges faced by Warner-Lambert in the early 1990s, resulted in reduced resources for R&D. Ron Cresswell, Chairman of Pharmaceutical Research, chose to focus his limited resources on a small number of promising molecules. One of the chemical compounds retained for development was a statin now known as Lipitor. At the time, Cresswell’s decision was viewed by some as “selling out” the company’s future, because he neglected products in early stages of development, future revenue-generators, in favor of late stage products that could soon earn money for the ailing pharmaceutical division.
The retention of the statin, Lipitor, was contentious at times since it appeared to be a “me-too” product that would enter a crowded market dominated by products from some of the most powerful pharmaceutical companies in the world. But, a Phase I study conducted in 1992 provided Warner-Lambert with an inkling that Lipitor could be a powerful cholesterol-lowering agent. LDL levels decreased nearly 50% in a trial involving healthy volunteers.[22] This was only first phase of tests in a long series of expensive clinical trials that Warner-Lambert needed to complete to gather data for the FDA (Exhibit 2). Additional evidence supporting the power of Lipitor was gathered in a 1994 Phase II trial which demonstrated that the drug could reduce cholesterol levels by more than 60%, exceeding the established capabilities of all competing products.[23] By the time Lipitor was ready for Phase III trials, Warner-Lambert researchers were confident that their drug was differentiable from existing statins because of its superior ability to lower cholesterol levels.
A large drug development hurdle that Lipitor faced was to get through the rigorous and lengthy FDA regulatory review process as quickly as possible. The time it takes the FDA to complete a drug review varies, but on average the agency completes them in 12 months.[24] But, if a new drug treats a serious or life-threatening condition or addresses an unmet medical need, the FDA will consider it for a fast track review (approval decision generally expected within six months). Warner-Lambert was under pressure to get its drug to market quickly, and data from a Phase II study led to a pivotal fast track strategy. In addition to conducting Phase III trials with people suffering from typical high cholesterol, the company decided to also run trials for familial hypercholesterolemia, a fatal hereditary condition resulting in exceptionally high cholesterol. A small minority of people suffer from this condition, and it was not clinically addressed by any of the competitive statins. “‘There wasn’t much of a downside risk,’ reported Robert Zerbe, Senior Vice President Worldwide Clinical Research, ‘If it didn’t work it [Lipitor] wouldn’t be any different than any of the others.’ And if it did, he said, ‘It would open up the possibility of priority review.’”[25] The results of the hypercholesterolemia trials justified an expedited review by the FDA and approval was granted for Lipitor (atorvastatin) just six months after submission of the New Drug Application (NDA).
As another part of Lipitor’s development strategy,
Warner-Lambert carried out head-to-head clinical trials against the leading
commercial statins at the request of the marketing group. This tactic is rarely
seen in the drug industry because if a new product fails to show significant
superiority to competitors, it will be very difficult to persuade physicians to
switch their patients to the new drug.
Incontrovertible evidence of superiority is, however, an extremely
desirable asset. Fortunately, Lipitor
was found to be superior to all commercialized statins. In the head-to-head
trials, Lipitor reduced elevated LDL cholesterol 40% to 60% across the full
dose range (10mg to 80mg) and reduced triglycerides by 19% to 40%. The best
selling competitive cholesterol reducer on the market, Zocor, decreased LDL
cholesterol by only about 40%.[26],[27],[28]
Yet another motive for the
aggressive push to market was the fact that Merck’s Mevacor was expected to
lose patent protection and go generic in 2001.
Warner-Lambert needed as much time as possible to shift patients and
physicians to its “new generation” drug, Lipitor, before generic Mevacor was available
at much lower prices.[29]
After his
1988 arrival at Warner-Lambert, Ron Cresswell launched several initiatives
instrumental in the development of Lipitor.
Cresswell sought, in many ways, to bring the research organization into
the modern world of pharmaceutical development and lay the foundation for a
strong future. He increased the emphasis on biotechnology, hired talent, and
built an experienced management team.
Moreover, Cresswell integrated regulatory affairs and clinical research
into the R&D organization with the intention of improving packages of
documentation submitted to the FDA. Cresswell also sought to involve marketing
earlier in the new drug development process.
“Both R&D and marketing had a clear sense of the product’s
[Lipitor’s] potential long before it received approval.” In addition, Cresswell
noted, “At the manufacturing level, we recognized some demanding parts of the
chemical synthesis and were able to build the kind of relationship early with
manufacturing that allowed us to make sure that the demands of that part of the
process didn’t hold the overall transition [Lipitor market entry] back.’”[30]
Lastly, Warner-Lambert management developed a new
compensation plan that uncapped performance incentives, thereby improving
overall employee morale.[31]
Based on the organizational
improvements and specific development strategies executed by Cresswell,
Warner-Lambert gained FDA approval in December of 1996 for Lipitor, launching
the product more than a full year ahead of many analyst predictions. Cresswell’s, and others efforts, contributed
to a turnaround in the Warner-Lambert pharmaceutical R&D organization.
Bringing Lipitor to Market
Lipitor Marketing Challenges
Warner-Lambert faced serious challenges in bringing Lipitor to market. Although clinical results indicated superior efficacy for Lipitor, the fact remained that it would be the fifth statin available to patients and physicians. One of Lipitor’s biggest challenges was to overcome the perception that it was a “me-too” product. Merck and Bristol-Myers Squibb, the incumbents, had proven products in the market. The companies marketing Zocor, Mevacor and Pravachol enjoyed strong relationships with key medical “opinion leaders” such as cardiologists and possessed large and experienced sales forces. How could Warner-Lambert, a smaller company, overcome the strong market positions held by the competitors?
Furthermore, Warner-Lambert was expecting to launch another major drug, Rezulin, at the same time as Lipitor. Many pharmaceutical companies view the first six months after a drug launch as the most critical time in a product’s life cycle. The initial adoption rate by physicians can often make or break a new product. A successful launch is critical to a product’s overall lifetime performance. To effectively launch one drug is a challenge but to simultaneously launch two, both with blockbuster potential, was daunting. How could Warner-Lambert successfully launch and market Lipitor?
Alliance with Pfizer
Warner-Lambert wanted to employ a “saturation” approach to selling Lipitor. This was essential because Lipitor faced a marketplace with millions of untreated patients and a medical community largely content with the drugs already available to treat high cholesterol. The intent of the “saturation” strategy was to have as many sales representatives as possible contacting physicians. As Anthony Wild, Warner-Lambert Pharmaceutical Sector President, explained “The more soldiers you have out there, the more guns, the more likely you are to achieve your ends.” Warner-Lambert clearly understood that the sales force was a key success factor in any drug’s performance, but a 1995 sales force deployment study conducted by consultants ZS Associates revealed that the company’s sales force was inadequate in size and focus to effectively launch Lipitor.[32]
Executives at Warner-Lambert decided that a partnership was necessary to address its constrained sales and marketing resources. They were faced with balancing the need for sales and marketing assistance with immediate demands to generate profits for the floundering pharmaceutical business. To find a suitable partner, Warner-Lambert focused its search on companies that did not have a competing cholesterol-lowering product. Of the five companies the field was narrowed to, Pfizer and Hoffman-LaRoche were the top two choices. Competition in the negotiation process improved the likelihood that Warner-Lambert would get a more favorable deal. At one point in the negotiations, a Warner-Lambert fax intended for Hoffman-LaRoche accidentally ended up in a Pfizer fax machine, which helped convince Pfizer that it needed to make a great offer in order to get the Lipitor deal. In the end, Pfizer was chosen by Warner-Lambert because of its marketing prowess, experience, contacts and credibility in cardiovascular medicine as well as recent successful drug launch experience. Moreover, unlike Hoffman-LaRoche, Pfizer was not then seen as a potential hostile acquirer.[33],[34]
Details of the co-marketing agreement (Exhibit 4) state that Pfizer paid $205 million in up front money and milestone payments for the rights to sell Lipitor. Pfizer also committed to splitting all future product expenses including advertising, promotion, sampling and sales force. Furthermore, Pfizer agreed to pay for half of any ongoing and planned clinical trials, which were reported to number more than 100 and involve 100,000 patients. In return, Pfizer would receive variable payments based on sales targets established in the agreement. Given the annual sales that Lipitor has reached, the arrangement indicates that Pfizer has been receiving 48% of net sales.[35]
Product Positioning
Lipitor was first and foremost marketed with a message of superior cholesterol lowering capability. Drug companies are restricted to only discussing therapeutic benefits of their drugs that have been approved by the FDA in what is termed an ‘indication.’ The head-to-head clinical trials proved that Lipitor was the most potent statin on the market for reducing elevated total cholesterol, LDL cholesterol and triglycerides over a range of comparable doses. Lipitor was the first statin approved for reducing triglycerides as well as the more common LDL cholesterol, making it an ideal drug for a broad range of patients. Although Pravachol and Zocor are effective at lowering high triglycerides, neither Bristol-Myers Squibb nor Merck possessed the triglyceride indication at the time of the Lipitor launch. Pravachol and Zocor promoted the fact that they were proven to reduce the risk of heart attacks and Zocor had the added indication of reducing deaths related to heart attacks. These were very powerful marketing messages that Lipitor could not exploit. Use of Lipitor most likely also prevents heart attacks and saves lives, but data proving this fact did not exist. Instead, Lipitor relied on the medical community perception that all statins possess the same capabilities to reduce heart attacks and death through their cholesterol lowering capabilities.[36]
The second piece of the Lipitor
positioning message revolved around its ease of use. Determining a patient’s optimal dose was not simple for
physicians to accomplish with existing statins. This entailed selecting a
starting dose, re-testing the patient’s cholesterol after having used the drug
for a period of time, adjusting the dose and repeating the cycle until the
proper dose was identified. The process
is inconvenient and time consuming.
Fortunately with Lipitor, 72% of patients reach their target LDL
cholesterol goal at the starting 10-milligram dosage thereby eliminating the need
for repetitive tinkering.[37],[38] As Carl Seiden, an analyst from
J.P.Morgan, put it, “The beauty of Lipitor’s positioning is that physicians can
remain ‘lazy’ by using the starting dose and then forgetting about it, but the
goals achieved with Lipitor will be greater than with any other statin.”[39]
Pricing Strategy
Warner-Lambert made the already
convincing Lipitor value proposition even better with a moderate pricing
strategy. The product was positioned
with a message of therapeutic superiority and ease of use, but priced lower
than the market leading competitors.
Figure 3 compares the average prescription price for each of the statins
at the time Lipitor entered the market.
Even after the incredibly positive reception and adoption of Lipitor
after launch, Warner-Lambert has maintained Lipitor average prescription prices
below its primary competitors’ prices.
Figure 3: Statin Average Prescription Pricing
Structure[40],[41]
|
Drug
Name |
1997 Average Prescription Price |
1999 Average Prescription Price |
|
Lescol |
$52 |
$50 |
|
Lipitor |
$84 |
$91 |
|
Pravachol |
$93 |
$105 |
|
Zocor |
$95 |
$125 |
|
Mevacor |
$125 |
$137 |
Promotional Tactics
An assortment of promotional
tactics were implemented by a marketing and medical team specifically dedicated
to Lipitor to build as much awareness in the medical community as possible.
Pre-launch efforts included a national cholesterol education program in
conjunction with the American Heart Association. The intent was to educate
physicians and patients that cholesterol needed to be treated more aggressively
than it was; they needed to “go for the goal” established in guidelines set by
the national cholesterol education program.
The campaign positioned Lipitor as the drug best suited for attaining
these targets. Additionally, Lipitor
was publicized in medical journals and at major medical conventions with
pre-market comparative efficacy data. Medical opinion leaders were also
recruited to use free samples of Lipitor immediately after the drug was
approved, but before it was formally launched.
These pre-marketing efforts succeeded-a week before Lipitor was
officially introduced, it already claimed 3% of all new cholesterol-lowering
drug prescriptions.[42],[43],[44]
Sales Force
Warner-Lambert and Pfizer outgunned the competition with the largest statin sales force. Between Warner-Lambert and Pfizer, more than 2,200 sales representatives were believed to be selling Lipitor during its launch in the U.S.[45] Lynn Alexy, Vice-President Warner-Lambert Cardiovascular Disease Team, described their physician selection. “In total, we targeted about 91,000 key prescribers – physicians either in the cardiology area, internists and general and family practitioners – who had shown a track record of writing cholesterol-lowering agents.”[46] By the end of the launch year, a total of 831,000 medical community contacts had been made on behalf of Lipitor, translating into a 29% share of voice in sales details which has been upheld through 1998.[47] A sales detail is simply a sales call on a doctor that results in the sales representative discussing the merits of a particular drug.
Figure 4: Cholesterol-Lowering Drug Physician
Details 1997[48]
The sales force further supported the Lipitor campaign through the distribution of free product samples. Warner-Lambert and Pfizer implemented an aggressive sampling program, distributing 7.3 million samples to physicians in the first year of launch for a share of voice of slightly over 23%.[49]
Lipitor Success
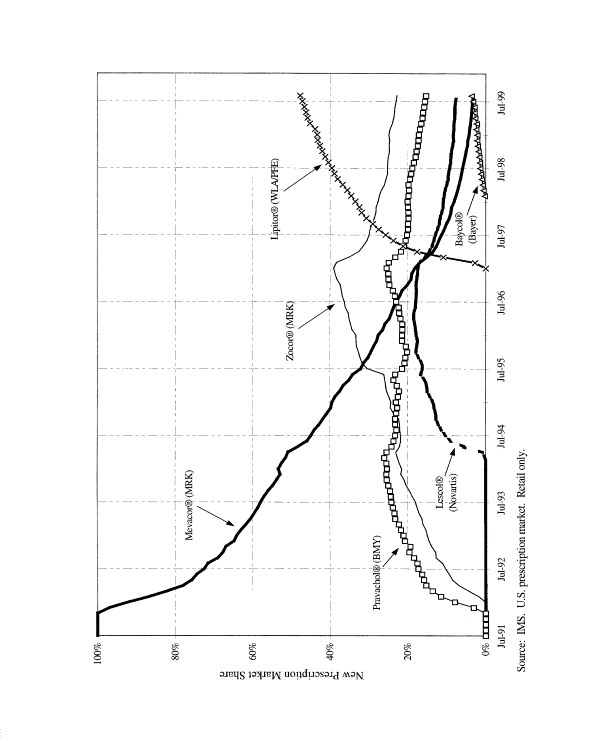
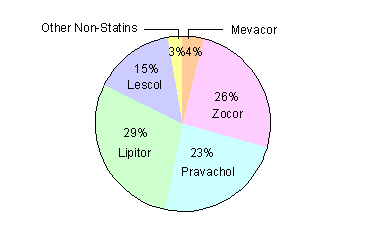
Lipitor is a blockbuster. Warner-Lambert and Pfizer have bragging rights to one of the most successful launches in the history of the pharmaceutical industry. After launching in January 1997, Lipitor reached $1 billion in domestic sales within its first 12 months on the market.[50] By the end of 1998, Lipitor was available for sale in 50 countries. Lipitor claimed U.S. market share leadership in the statin drug class in October 1997 with a 30% share of all new statin prescriptions (Exhibit 5).[51] Lipitor has continued to take share, finishing 1998 with 34% of the entire cholesterol-lowering new prescription market (Exhibit 6).
Hemant Shah, President of HKS &
Company, a health care analysis firm, reiterated the sentiments of many when he
said, “In my two decades of experience within the pharmaceutical industry, I
have never witnessed the launch of a fifth comparable drug to treat a
non-symptomatic disease take off as rapidly as Lipitor has.”[52] The drug’s success even caught
Warner-Lambert by surprise.
Manufacturing executives admitted that the market demand was so strong
that they exhausted what they thought was a 3-month drug supply virtually
overnight. The company was forced to
purchase a fully operational manufacturing facility just months after Lipitor
launched in an effort to keep up with demand.[53]
Industry analysts reported that Warner-Lambert internal first year Lipitor
sales expectations were set at only $100 million just prior to launch.[54]
Lipitor Next Steps
Future Lipitor Strategies
To date, Lipitor has benefited from the DTC advertising campaigns of its competitors. Both Merck and Bristol-Myers Squibb have spent tens of millions of dollars in DTC advertising for Zocor and Pravachol only find that they were driving more patients to physicians, but that they were getting prescriptions for Lipitor. Warner-Lambert is now dropping its hat into the DTC ring with Lipitor as a means of expanding the entire statin market. Print and television ads were introduced in February 1999. Using the tagline, “The lower numbers you are looking for,” Warner-Lambert is expecting to encourage patients to have their cholesterol checked with the hope that they will eventually be prescribed Lipitor.[55]
Warner-Lambert and Pfizer have been
leveraging their relationship in an effort to identify Lipitor extensions. One
concept is for a single product that concurrently treats high cholesterol and
hypertension. The combination pill would include Lipitor and Pfizer’s calcium
channel blocker, Norvasc. Although this
potential extension was publicly shared, the recent merger battles bring into
question whether this is a still a viable joint development program.[56]
Finally, Warner-Lambert has quietly admitted to examining the regulatory hurdles in selling Lipitor over-the-counter. A conversion from prescription to over-the-counter (OTC) rarely happens before a drug has lost patent protection. However, it appears that Warner-Lambert may be interested in pre-empting its patent expiry since protection will continue through 2010 for Lipitor.[57]
Future of Lipitor
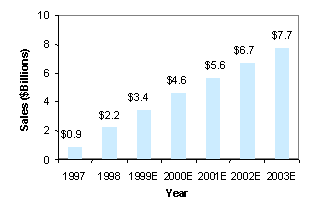
Pharmaceutical industry analysts widely interpreted Pfizer’s hostile attempt to take over Warner-Lambert as motivated by concern over the AHP merger’s potential to affect the Lipitor co-marketing agreement. Worldwide Lipitor sales for 1999 are approaching $4 billion, only three years after introduction. Figure 5 illustrates one analyst’s Lipitor sales projections through 2003, but sales are predicted to reach $10 billion before 2010 when patent expiration is expected.[58] Pfizer claims almost half of the drug’s annual profits, making Lipitor an invaluable part of Pfizer’s drug portfolio today and in the future.
Figure 5: Lipitor Worldwide Sales
Projections[59]
As industry analysts hotly debated the fight for Warner-Lambert through the media, the players in the competition jockeyed to better position themselves for the impending contest. For de Vink to be battle-ready he needed to solidify his position on some important questions. Can Warner-Lambert continue to sell Lipitor on its own or does Pfizer still have something unique to provide? How much is Lipitor worth?
Exhibit 1: U.S. Market Share Top 20 Pharmaceutical Companies, 1998
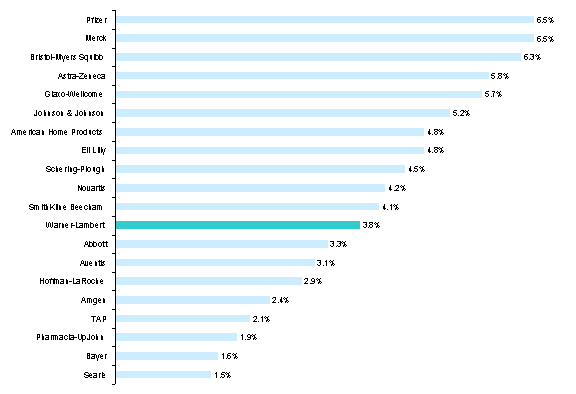
Source: U.S.
Prescription Product Market, Year In Review 1998, IMS Health
Exhibit 2: U.S. Drug Development Process
DISCOVERY AND DEVELOPMENT
In the discovery phase, pharmaceutical companies employ thousands of scientists to search for compounds capable of affecting disease. While this was once a process of trial and error and serendipitous discovery, it has become more rational and systematic through the use of more sophisticated technology. But safety remains the paramount concern. Pharmaceutical companies and Federal Drug Administration (FDA) take extraordinary measures to ensure the safety of all approved prescription drugs. From discovery through post-marketing surveillance, drug sponsors and FDA share an overriding focus to ensure that medicines are safe and effective.
Once an entity has been identified as a potential drug candidate, FDA and industry follow elaborate scientific procedures to evaluate the safety and efficacy of the candidate. The procedures are divided into four specific stages: pre-clinical safety assessment, pre-approval safety assessment in humans, safety assessment during FDA regulatory review, and post-marketing safety surveillance.
PRE-CLINICAL ASSESSMENT
The relative safety of newly-synthesized compounds is initially evaluated in both in vitro and in vivo tests. If a compound appears to have important biological activity and may be useful as a drug, special tests are conducted to evaluate safety in the major organ systems (e.g., central nervous, cardiovascular, and respiratory systems). These pharmacology studies are conducted in animals to ensure that a drug is safe enough to be tested in humans.
An important goal of these pre-clinical animal studies is to characterize any relationship between increased doses of the drug and toxic effects in the animals. Development of a drug is usually halted when tests suggest that it poses a significant risk for humans—especially organ damage, genetic defects, birth defects, or cancer.
PRE-APPROVAL ASSESSMENT IN HUMANS
A drug sponsor may begin clinical studies in humans once FDA is satisfied that the pre-clinical animal data does not show an unacceptable safety risk to humans. It takes many years for a clinical development program to gather sufficient data to prepare a New Drug Application (NDA) seeking FDA regulatory review to market a new drug.
Every clinical study evaluates safety, regardless of whether safety is a stated objective. During all studies, including quality-of-life and pharmacoeconomic studies, patients are observed for adverse events. The average NDA for a novel prescription drug is based on almost 70 clinical trials involving more than 4,000 patients—more than twice the number of trials and patients for the NDAs submitted in the early 1980s. Clinical studies are conducted in three stages:
Phase I: Most drugs are evaluated for safety in healthy volunteers in small initial trials. A trial is conducted of a single dose of the drug, beginning with small doses. If the drug is shown to be safe, multiple doses of the product are evaluated for safety in other clinical trials.
Phase II: The efficacy of the drug is the primary focus of these second-stage trials, but safety also is studied. These trials are conducted with patients instead of healthy volunteers; data are collected to determine whether the drug is safe for the patient population intended to be treated.
Phase III: These large trials evaluate safety and efficacy in groups of patients with the disease to be treated, including the elderly, patients with multiple diseases, those who take other drugs, and/or patients whose organs are impaired.
FDA REVIEW
A sponsor submits an NDA to the FDA for approval to manufacture, distribute, and market a drug in the U.S. based on the safety and efficacy data obtained during the clinical trials. In addition to written reports of each individual study included in the NDA, an application must contain an integrated summary of all available information received from any source concerning the safety and efficacy of the drug.
The FDA usually completes its review of a "standard" drug in 10 – 12 months. One hundred and twenty days prior to a drug’s anticipated approval, a sponsor must provide the agency with a summary of all safety information in the NDA, along with any additional safety information obtained during the review period.
While the FDA is approving drugs more expeditiously, the addition of 600 new reviewers made possible by the user fees paid by pharmaceutical companies has enabled the agency to maintain its high safety standards. Over the years, the percentage of applications approved and rejected by FDA has remained stable. Two decades ago, 10 – 15 percent of NDAs were rejected—the same as today.
POST MARKETING SURVEILLANCE
Monitoring and evaluating a drug’s safety becomes more complex after it is approved and marketed. Once on the market, a drug will be taken by many more patients than in the clinical trials and physicians are free to use it in different doses, different dosing regimens, different patient populations, and in other ways that they believe will benefit patients. This wider use expands the safety information about a drug.
Adverse reactions that occur in fewer than 1 in 3,000 – 5,000 patients are unlikely to be detected in Phase I – III investigational clinical trials, and may be unknown at the time a drug is approved. These rare adverse reactions are more likely to be detected when large numbers of patients are exposed to a drug after it has been approved.
Safety monitoring continues for the life of a drug. Post-marketing surveillance is a highly regulated and labor-intensive global activity. Even before a drug is approved, multinational pharmaceutical companies establish large global systems to track, investigate, evaluate, and report adverse drug reactions (ADRs) for that product on a continuing basis to regulatory authorities around the world.
Direct Excerpt: Pharmaceutical Industry Profile 1999, PhRMA
Exhibit 3: Warner-Lambert Sales By Business Segment 1998
|
Business
Segment |
% WorldWide Sales |
Worldwide Sales ($ Millions) |
U.S. Sales ($ Millions) |
% Profit Margin |
|
Pharmaceuticals |
57% |
$6,191 |
$4,232 |
22% |
|
Lipitor |
20% |
$2,184 |
$1,645 |
- |
|
Consumer Products |
25% |
$2,721 |
$1,475 |
18% |
|
Confectioneries |
17% |
$1,889 |
$661 |
11% |
|
Total |
|
$10,801 |
$6,368 |
|
Sources: PaineWebber Drug Universe, October 11, 1999
SG Cowen Pharmaceutical Industry Pulse, March 1999
Exhibit 4: Warner-Lambert Summary of Lipitor Agreement with
Pfizer Inc
SUMMARY OF KEY TERMS OF LIPITOR ARRANGEMENTS
· Covers most major countries other than Japan & France; primarily co-promotion; 10 year term from launch
· $205 million from Pfizer in upfront & milestone payments for rights to Lipitor (already received)
· One quid pro quo product from Pfizer, chosen from among three listed in agreement, on terms to be negotiated; $30 million payable at rate of $6 million per year beginning in 2002 if no quid selected
· U.S. payments to Pfizer tied to baseline sales:
|
Agreement Year |
Baseline Sales |
|
1 |
$263
million |
|
2 |
$337
million |
|
3 |
$457
million |
|
4 |
$541
million |
|
5 |
$588
million |
|
6 |
$612
million |
|
7 |
$651
million |
|
8 |
$688
million |
|
9 |
$763
million |
|
10 |
$753
million |
Note: Agreement Year
1 ended March 31, 1998
|
|
% OF NET SALES
("Standard" Definition) |
||
|
Up
to 50% of Baseline |
50%
to 100% of Baseline |
Over Baseline |
|
|
Year 1 through 5 |
13.35 |
31.15 |
48.00 |
|
Years 6 and 7 |
13.35 |
31.15 |
44.50 |
|
Year 8 |
13.35 |
22.25 |
31.15 |
|
Year 9 |
13.35 |
13.35 |
31.15 |
|
Year 10 |
8.90 |
8.90 |
31.15 |
· Pfizer pays 50% of all Product Expenses for years 1-7 and 35% of all Product Expenses for years 8-10. Product expenses include the following.
§ Marketing, A&P, sampling
§ Training and communications materials
§ Product Lifecycle Plan Studies (clinical, pharmacoeconomic, etc.)
§ Regulatory filings
§ Not detailing (each party performs and pays for its own details)
§ Not cost of goods or distribution
· In International co-promotion countries, % of Net Sales payable to Pfizer ranges from 26.4% up to 44% (when market share hits 38%)
· In International co-promotion countries, Pfizer pays 50% of all Product Expenses and performs/pays for 50% of details (except Germany: 2/3 Warner-Lambert, 1/3 PFE)
· In International license countries, Warner-Lambert sells bulk tablets to Pfizer at 28% of Net Sales (and Pfizer covers all of its other costs)
TERMINATION PROVISIONS
· Both U.S. and International co-promotion arrangements can be terminated at the option of Warner-Lambert at the beginning of Agreement Year 6 (or at the beginning of Agreement Year 4 upon a Change of Control of Warner-Lambert)
· One year advance notification
· Any or all territories
· If terminated...
§ Pfizer continues to fund its portion of covered Product Expenses
§ Pfizer withdraws sales force support
§ Warner-Lambert pays Pfizer 75% of the amount due under the agreements
§ Pfizer is not obligated to pay annual quid option cash payments; years 6-10 $6MM each year
§ Both parties have the right to terminate for material breach (and above provisions not applicable)
This summary is intended to provide readers with a brief, clear explanation of the agreement's most salient points. The full texts of the five agreements are available at www.warner-lambert.com
Source: Warner-Lambert
Exhibit 5: U.S. Statin Market Share of New Prescriptions 1991-1999
Source:
Salomon Smith Barney, Scrip Picture Book, October 8, 1999
Exhibit 6: Cholesterol Lowering Prescription Drug Market Share 1998
|
Drug |
U.S. New Prescription Market Share (%) |
Worldwide Sales ($millions) |
|
Lipitor |
34% |
$2,185 |
|
Baycol |
1% |
$125 |
|
Zocor |
24% |
$3,945 |
|
Mevacor |
6% |
$745 |
|
Pravachol |
16% |
$1,643 |
|
Lescol |
8% |
$612 |
|
Other Non-Statins |
12% |
|
Note:
Baycol launched by Bayer /SmithKline Beecham 1998
Sources: JP Morgan, Prescription Pad, November 18, 1999
Morgan Stanley Dean Witter, Novartis: Keeping the Faith, February 3, 1999