Case 13: P.T. Pharma Indonesia
"Hey mister, nice watch!" The call of a beach peddler rudely roused Tom Auckland from the siesta he was enjoying while on a brief vacation with his family at the Santika Beach Resort on Bali. The intrusion brought him back to reality in more ways than one. He would be confronted with multiple problems on his return to Jakarta where he was posted as president director of P.T. Pharma Indonesia. The vendor, with his case of cheap copies of expensive watches, reminded Tom of one of the many issues he had to address in his strategic plan requested by corporate headquarters. Among other things, he had to recommend ways to cope with the endemic problems of look-alike brand name pharmaceuticals, which were rampant on the Indonesian market. He also had to explain how the Indonesian market should be viewed in light of Pharma's new strategy of becoming a truly global company, rather than simply a United States company with international activities.
Pharma had started in the Indonesian market in 1973 with a 70/30 percent joint venture arrangement, as is common in host countries seeking to control their markets by providing members of the local establishment with lucrative business opportunities. The beginning was promising. Unlike many other foreign investors, Pharma had exceedingly good luck with the choice of a local partner. Originally, Pharma was attracted to Indonesia by the size of the potential market, some of whose 180 million people had recently come into money due to the incipient oil and gas boom in the 1970s.
The company began as a marketing operation with a packaging plant, where the imported active ingredients of various pharmaceuticals were put into vials, tablets and capsules, and subsequently sold locally. In 1986 Pharma's plant was financed largely with an injection of capital sources from abroad.
Operating through a joint venture was only one of the many Indonesian requirements for foreign firms. Import substitution was also an important government objective. All foreign joint venture companies in the pharmaceutical industry had to build chemical plants in Indonesia and produce at least one active ingredient locally. This requirement posed one of Tom's current major problems. Pharma's chemical plant, like the facilities of most of its foreign competitors, was designed, because of technological requirements, for a sales volume that had never materialized. Moreover, the government did not impose the same requirement on domestically-owned producers, which aggravated the problem considerably. Domestic competitors were able to import the active ingredients from least-cost sources abroad. This preferential treatment of domestic firms was reinforced by government promotion of cheap generics in order to aggressively lower the cost of drugs to the final consumer. At the same time, the government provided little assistance in terms of patent and trademark protection—the relevant legal framework in Indonesia was underdeveloped and enforcement was largely ineffective. Consequently, Pharma's products were copied by domestic entities, not unlike watches and other brand name consumer products.
The expense of operations due to excess plant capacity contributed to the disproportionately high cost of Pharma's pharmaceuticals, leaving the company in virtually the same position as the rest of its foreign competitors. The market share numbers tell the story. Whereas in 1985 the market share of foreign investors was 52%, by the end of 1991 the share of the market of pharmaceutical companies owned by foreign investors would be reduced to 40%, according to optimistic estimates. In the late 1980s, most foreign competitors had taken advantage of the new spirit of economic liberalization in Indonesia and had allowed production activities at their chemical plants to run down, while they substituted less expensive imports. Still, foreign firms had to maintain their chemical plants to comply with Indonesian requirements, constituting a continuing drain on profits. The special requirements for producing at least one active ingredient locally necessitated a big capital commitment, and maintaining sterile conditions in pharmaceutical plants involved high operating costs, even though output had been reduced to a minimum over time. In addition, the Indonesians had recently discovered environmental concerns and were beginning to impose new environmental regulations, particularly on foreign companies (which domestic competitors managed to ignore).
Overall, the current operating environment for foreign pharmaceutical companies in Indonesia continued to be adversely affected by government policies precluding at least two obvious remedies. One potential solution to the problem of excess plant capacity would be to allow foreign joint ventures to produce the active ingredients for other foreign competitors, so-called "contract" or "toll" production. Another potential improvement would be to permit the plants owned by foreign companies to produce over-the-counter (OTC), or nonprescription drugs, particularly aspirin, headache pills and cough syrup. However, the Indonesian government had, with few exceptions, mandated OTC production to locally-owned firms. Consequently, these possible remedies did not look like effective cures for P.T. Pharma Indonesia.
Complicating matters further was the possibility that, as rumor had it, high officials in the health care sector of the government owned substantial shares in domestic pharmaceutical firms. It was even alleged that some local firms, owned by close relatives of officials at the highest levels of government, thrived by importing brand name products (including Pharma's) from all over the world, wherever they could be sourced most inexpensively. Because of the impact of national regulation on drug prices in a number of countries, there was ample opportunity for such gray market activities. In summary, Pharma Indonesia had high costs and confronted three sets of competitors: (1) gray market importers of Pharma's own products; (2) domestic imitators; and (3) domestic competitors producing the same products as generics, but without the same costly quality-control constraints and requirements for domestic content.
In developing P.T. Pharma Indonesia's business strategy, there were 101 issues that needed to be addressed, none of which was easy. However, two questions were of strategic importance, particularly in the spirit of Pharma's new motto to "think globally—act locally." First, what were Pharma's core competencies that might lead to a competitive advantage in some pharmaceutical market segments in Indonesia, and allow Pharma to achieve an appropriate return, given the financial and managerial resources employed? Second, what strategy would take into account the existing nature and potential future development of the Indonesian market for pharmaceuticals? Pharma's strategy with respect to Indonesia had to be consistent with both the company's opportunities in the local market and its global strategy. After all, at best, Indonesia accounted for only $10 million in sales in 1991, representing approximately 3% of Pharma's total worldwide sales. To what extent then could Pharma afford to devote resources, especially scarce top management time, to such a small and troublesome market? This question needed to be addressed in light of the fact that Indonesia was the fifth largest market in the world by population, and had shown sustained growth in personal income, which put it in the ranks of emerging markets with real growth rates of output in the vicinity of 7% per annum since 1988.
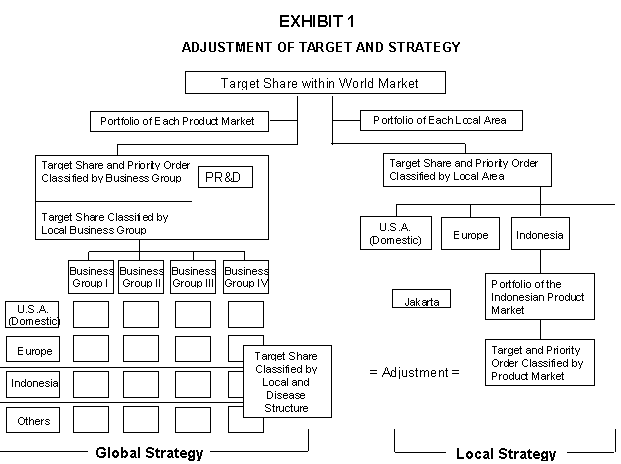
Tom Auckland paused. He pulled from his beach bag the Pharma Strategic Planning Handbook and reviewed the chart titled "Adjustment of Target and Strategy." "How are we going to fashion a report on the complexities of the Indonesian market to fit the corporate planning framework?" he pondered.
Appendix 1
The Pharma Company
After a long history, Pharma had reached its current position as seventeenth in the global pharmaceutical market and ninth in the U.S. market (as measured by 1988 sales). Pharma started as a pill-producing company in the late 1880s, but, like its competitors, turned to research and development as a major source of competitive strength in the inter-war period. Through a mixture of prudent management decisions and the vagaries of the research process, Pharma developed core competencies focusing on the following: (1) steroids, anti-inflammatory and analgesic agents; (2) antibiotics; and (3) pharmaceuticals operating on the central nervous system, in particular Xandu, an anti-anxiety agent, and Snoozion, which combats chronic insomnia. More recently, the search for a remedy for high blood pressure resulted in the accidental discovery of Regain, licensed as an effective treatment for male potency.
Over time, Pharma's research activities were reorganized to focus on the following five principal areas:
1. The central nervous system.
2. Cancer and infectious disease, including antibacterials, antivirals and AIDS research.
3. Cardiovascular problems.
4. Anti-inflammatory remedies.
5. Metabolic disorders.
As part of Pharma's new emphasis on globalization, the company also integrated the research and medical affairs divisions into a single worldwide operation, newly named Pharma Laboratories. The objective of this new setup was to coordinate the development of major products for the global market in order to mitigate the rising cost of pharmaceutical research and to shorten development time. Pharma's major lab locations are in Columbia, OH, Japan and the United Kingdom. Pharma recognized the need to develop products at different locations to benefit from the cross-fertilization of scientific knowledge internationally. To facilitate the registration process for new products, a degree of international diffusion had become increasingly necessary to provide access to medical opinion leaders. This strategy also helped in marketing new products once they were licensed.
Pharma's researchers were under considerable pressure to develop significant new products because several of the company's major money-making pharmaceuticals were due to lose patent protection. More specifically, Xandu and Snoozion, which in recent years had produced 30% of Pharma's profits and 15% of the company's sales, would lose patent protection in 1993. While there were some promising products in the pipeline, the delays and the uncertainties of the FDA approval process made this a very crucial period for the company. Consequently, a number of important changes had been implemented and more were being considered. Pharma had followed the industry trend by forming several strategic alliances with both domestic and foreign competitors. Indeed, Pharma had a history of licensing products externally, dating back to 1974 when Motrin was licensed from Boots in the U.K. Pharma had also entered into co-marketing agreements and had established several joint ventures to produce and market specific products. Furthermore, there were recurrent rumors emanating from Wall Street that the company itself was on the market, although Pharma management continued to deny this and similar radical alternatives.
Meanwhile, the process of globalization also shaped Pharma's marketing and sales functions. A Worldwide Pharmaceutical Marketing Division had been created to provide better marketing worldwide, rather than just in the U.S. This division consisted of the following four business groups: (1) Anti-Inflammatory and Critical Care; (2) Cardiovascular Diseases; (3) Infectious Diseases, Reproductive Medicine and Oncology; and (4) Central Nervous System Products. Within each of these Business Groups, there were directors responsible for specific diseases or product categories. They in turn supervised mangers, who were responsible for marketing specific products. These managers had responsibility for marketing products worldwide, abroad or in the U.S., depending on the size of the market involved. To accomplish this task, they used marketing teams for individual drugs. These teams planned product marketing on a worldwide basis, and provided inputs toward global strategic product plans, which were then integrated into business plans.
Using a matrix form of organization, Pharma also established seven regional management marketing positions. These regional marketing managers reported to the division vice-president for their particular region. The regional marketing managers were responsible for coordinating the marketing plans for their specific regions with the product managers, as well as with the heads of individual subsidiaries. These regional marketing managers were also responsible for activities that affected their whole region, including regular regional medical meetings. They also assisted individual subsidiaries in implementing major new initiatives.
Pharma's globalization efforts in the marketing area required coordination of all marketing activities throughout all the markets where Pharma operated. For example, the advertising function provided professional expertise in all marketing subsidiaries worldwide. Along the same lines, the company had established a central clearinghouse for media buying worldwide. As part of the globalization effort, an Executive Information System (EIS) had also been developed.
Pharma's strategic challenges and new organizational structure also defined the planning parameters for P.T. Pharma Indonesia. Clearly, people at corporate headquarters were preoccupied with more immediate issues than a small, strange and troublesome market. Moreover, some of Pharma's core competencies did not lend themselves to developing a competitive advantage in Indonesia. The Indonesian people, with a per capita income of U.S. $1,822 (1988), were not a promising market for pharmaceuticals such as Regain, or products to soothe the central nervous system. Very few Indonesians have the luxury of caring about male potency, anxiety attacks or insomnia. (Exhibit 1–Adjustment of Target and Strategy–summarizes the strategic planning process with respect to Indonesia.)
On the other hand, Pharma's product lines of anti-inflammatory agents and antibiotics might have considerable potential in Indonesia. And, some years ago, Pharma's research expertise on steroids yielded a product called Estrena, a liquid contraceptive with long-term effectiveness, which had already played a significant role in the Indonesian government's concerted efforts to reduce population growth. Government family planning efforts had been quite successful in Indonesia. At the same time, supplying products for such government-run programs, which are highly politicized, continued to present special challenges. Pharma needed to know how to approach government officials, especially high-ranking officials, how to entertain them and how to maintain harmonious relationships with them.
Moreover, Estrena illustrated the difficulties involved in developing global marketing strategies for pharmaceutical products, which are subject to licensing by national governments. Animal tests with Estrena in the U.S. showed that beagles who received large doses developed breast cancer. Subsequently, the FDA refused to license Estrena for use in the U.S. However, authorities in the U.K. and many other European countries did not believe that tests on beagles were conclusive as to the effect on humans, and therefore continued to license the product.
Beyond these so-called "ethical drugs," or drugs available only with a doctor's prescription, Pharma had several lines of OTC products with potential in the Indonesian market. Of particular importance were: multivitamins, antibiotics, Motrin and other analgesics, Diarex and other anti-diarrheal pharmaceuticals.
Appendix 2
Indonesia’s Pharmaceutical Industry
With a population of around 180 million people, Indonesia has been regarded as a huge potential market. The prospects of a big market have prompted domestic as well as many foreign investors to develop industries producing goods and services. The pharmaceutical industry represents one of these sectors. However, in Indonesia, as in other large developing countries, there has been a big gap between "potential" and its realization. This applies also to the pharmaceutical industry.
Not all companies operating in Indonesia have profited from the increased consumption of pharmaceutical products. Many firms are beset with difficulties amid fierce competition; however, along with the economy, the pharmaceutical industry in general has grown.
Economic Performance
In the 1970s, Indonesia became a substantial exporter of oil and gas, qualifying the country for membership in OPEC. It benefited greatly from the rise in oil prices during that decade and correspondingly suffered as oil prices decreased. Indonesia's economy began to revive after the severe recession hit the oil-producing economies in the mid-1980s. Subsequently, in 1988 the country's economic growth rate increased to 5.7%. Since that time, real growth has continued vigorously, albeit accompanied by high levels of inflation. (See Table 1.)
Table 1
Indonesia's Economic Growth and Inflation Rate.
1985–1990
|
Year |
Economic Growth (%) | Inflation
Rate (%) |
| 1985 | 2.5 | 4.3 |
| 1986 | 4.0 | 8.8 |
| 1987 | 3.6 | 8.9 |
| 1988 | 5.7 | 5.5 |
| 1989 | 7.4 | 6.6 |
| 1990 | 7.4 | 9.5 |
| 1991 | 5.6 | 9.5 |
Source: Indocommercial Business Report, Dec. 1991.
Natural Conditions and Population
Indonesia consists of 13,677 islands, with a total area of 1.92 million square kilometers and with a land mass of 919,433 square kilometers. It is a tropical country with two seasons: the rainy season from October to March, and the dry season from April to September. The temperature ranges from 22°C to 32°C with humidity ranging between 75% and 90%.
According to the Indonesian Central Bureau of Statistics, the country had a population of 148 million in 1980, which grew to 164.6 million in 1985. In 1989, the population was estimated at 179.8 million. The population is expected to increase to 222.7 million in the year 2000. Indonesians 0–24 years old made up 58% of the total population in 1985. In 1990, the number of people in this age group was 56% of the total population, possibly the result of government population policies.
Java is the most densely populated island in Indonesia. With an area of 132,187 square kilometers, or 14.4% of Indonesia's total land mass, Java had a population of 105.6 million (60.2% of total population) in 1988. This gave it a population density of 812 people per square kilometer, whereas Sumatra had 36.2 million or 20.6% of total population, with a density of 78 people per square kilometer, a fact representing just one dimension of Indonesian diversity.
Common Diseases and General Health
A household health survey showed that 11.5% of the Indonesian population was sick in 1980. A subsequent 1986 survey revealed a figure of 8.3%. Neither survey revealed a significant change in the percentage of sick among the total number of babies from 15.7 in 1980 to 16.6 in 1986. However, the incidence of ill health among people aged 55 years and above decreased sharply from 25.2% in 1980 to 15.1% in 1986.
About 5.2% of the babies born in 1983 suffered from low birth weight. The figure changed little in 1986. The number of children suffering from poor nutritional condition among those aged below five years was 16% in 1978 and 11.1% in 1987.
In Indonesia, infectious diseases are most common, such as tuberculosis, gastritis, malaria, morbilli (a tropical version of measles, with high fever, mostly suffered by children) and internal diseases. A survey conducted by the Central Bureau of Statistics in 1987 showed that out of 167.6 million Indonesians, 1.5% suffered from internal diseases, 1.3% suffered from morbilli, 1.3% suffered from malaria, .5% suffered from gastritis, .3% from TB and 9.1% from other diseases. In 1987, 24.2% of the sick people lived in urban communities; rural communities accounted for the rest.
The crude death rate (CDR) in Indonesia is declining. In 1971, the CDR was recorded at 18.7 per thousand people. The figure declined to 12.5 in 1980 and to 7.5 by 1990.
Health Services
Most hospitals in Indonesia are located in Java, along with the bulk of the population. According to the health ministry, Java has 772 hospitals, representing 52.5% of the country's total hospitals. Combined, these hospitals have a total of 61,278 beds or 55.6% of the total number of hospital beds in the country. All municipal towns of subdistrict areas have health centers. In 1986, there were 5,553 health centers, 16,636 auxiliary health centers and 4,470 mobile health centers in Indonesia.
The Indonesian government encourages the development of privately owned hospitals. In 1985/1986, the number of privately own hospitals was recorded at 773, with a total of 34,039 sick beds. In 1988/1989, the number of hospitals rose to 860, with a total of 38,670 sick beds. Government expenditures for the health sector have risen substantially. A 23.6% increase to Rp. 137,051 million was recorded in the development budget for the health sector in fiscal 1988/1989. In previous years, the health development budget was only Rp. 110,845 million.
Overview of the Pharmaceutical Industry
The pharmaceutical industry in Indonesia has been well-established compared with other industries in the country. It has particularly developed since 1955 when there were only seven pharmaceutical plants in the country. It then began to grow significantly in the 1960s, especially after the introduction of the Foreign Capital Investment Law in 1967 and the Domestic Capital Investment Law in 1968. Already in 1966 this number had risen to 130. The number of plants continued to increase after the introduction of the Investments Law. By 1975 there were 230 factories, in 1984 there were 273 and in 1988 the figure rose to 285.
The development of the pharmaceutical industry was paralleled by other related businesses, such as wholesalers. In 1969 there were 500 wholesalers of pharmaceutical products, 840 dispensaries and 120 drugstores; however, in 1988, there were 1,081 pharmaceutical wholesalers, 2,211 dispensaries and 1,984 drugstores. (See Table 2.)
Table 2
Development of Pharmaceutical Businesses
in Indonesia 1985–1988.
| Year | Pharm. Plants | Wholesalers | Dispensaries | Drugstores | Total |
| 1985 | 287 | 910 | 1672 | 1201 | 4070 |
| 1986 | 285 | 912 | 2051 | 1500 | 4748 |
| 1987 | 285 | 928 | 2163 | 1650 | 5041 |
| 1988 | 285 | 1081 | 2211 | 1984 | 5561 |
Source: G.P Farmasi, 1988, Indonesian Association of Pharmaceutical Companies.
Given the distribution of population, skills and infrastructure, it is not surprising that most of Indonesia's pharmaceutical plants are located in Java. In 1955, 243 or 88.7% of pharmaceutical plants operating in Indonesia were located in Java, with the largest number in Jakarta and West Java. These two regions had a total of 159 factories in 1985, East Java had 49, Central Java had 31 and Yogyakarta had 4. Outside Java, North Sumatra topped other regions with 16 factories.
In 1988, the number of pharmaceutical factories in Java rose to 256 or 89.8% of the country's total number of factories. (See Table 3.)
Table 3
Pharmaceutical Factories and their Locations 1985–1988.
| Location | Number of Factories | |||
| 1985 | 1986 | 1988 | ||
| Jakarta | 74 | 77 | 78 | |
| West Java | 85 | 83 | 88 | |
| Central Java | 31 | 32 | 32 | |
| Yogyakarta | 4 | 4 | 4 | |
| East Java | 49 | 52 | 54 | |
| Aceh | 1 | 1 | 1 | |
| N. Sumatra | 16 | 16 | 15 | |
| W. Sumatra | 2 | 2 | 2 | |
| Jambi | 1 | 1 | 1 | |
| S. Sumatra | 6 | 6 | 4 | |
| Lampung | 1 | 1 | 1 | |
| W. Kalimanta | 1 | 1 | 1 | |
| N. Sulawesi | 1 | 1 | 1 | |
| S. Sulawesi | 2 | 2 | 2 | |
| Bali | 1 | 1 | ||
| Total | 274 | 280 | 285 | |
Source: G.P. Farmasi, 1988, Indonesian Association of
Pharmaceutical Companies.
Indonesian "national" companies in 1988 were recorded at 244 and foreign joint venture companies made up the remaining 40. The 40 foreign joint venture companies, although small in number, have established a strong market share in Indonesia. Most of them are multinational companies. (See Table 4.)
Table 4
Foreign Joint Venture Pharmaceutical Firms.
Pharmaceutical Manufacturer Location
P.T. Abbott Indonesia West Java
P.T. Bayer Indonesia Jakarta
P.T. Beecham Pharmaceutical Jakarta
P.T. Beidersdorf Indonesia East Java
P.T. Bristol Myers Indonesia West Java
P.T. Burroughs Wellcome Indonesia West Java
P.T. Carlo Erba Farmatalia West Java
P.T. Ciba-Geigy Pharma Indonesia Jakarta
P.T. Dmex Indonesia Jakarta
P.T. Eisai Indonesia West Java
P.T. Essex Indonesia/Schering USA East Java
P.T. Glaxo Jakarta
P.T. Hoechst Pharmaceutical Jakarta
P.T. Hudson Indonesia West Java
P.T. ICI Farmasi Indonesia East Java
P.T. Johnson & Johnson Indonesia East Java
P.T. Medifarma Laboratories Jakarta
P.T. Meiji Indonesia East Java
P.T. Merck Indonesia Jakarta
P.T. Merrel Dow Pharm Jakarta
P.T. Nattermann Indonesia West Java
P.T. Nicholas Laboratories Ind. Jakarta
P.T. Nordmark Jakarta
P.T. Organon Indonesia Jakarta
P.T. Otsuka Indonesia Jakarta
P.T. Parke-Davis Jakarta
P.T. Pfizer Indonesia Jakarta
P.T. Pfrimer Infusol Indonesia West Java
P.T. Pharma Indonesia West Java
P.T. Rhone Poulenc Indonesia West Java
P.T. Richardson Vicks Indonesia Jakarta
P.T. Roche Indonesia West Java
P.T. Salonpas Indonesia Jakarta
P.T. Sandoz Biochemie Farma East Java
P.T. Schering Indonesia Jakarta
P.T. Squibb Indonesia West Java
P.T. Sterling Product Indonesia West Java
P.T. Takeda Indonesia West Java
P.T. Tanabe Abadi West Java
P.T. Upjohn Indonesia West Java
P.T. Warner Lambert Indonesia West Java
Source: P.T. Corinthian (CIC), 1990.
Characteristics of Production and Products
Pharmaceutical factories in Indonesia are "formulation" factories since most basic (active) ingredients are imported. So far the country has produced a limited quantity and few types of basic (active) ingredients.
After a lengthy period of government policies promoting import substitution, companies have been able to meet all essential medical needs in Indonesia. Since the late 1980s, only a few products have needed to be imported. According to the Indonesia Association of Pharmaceutical Companies, there were 9,246 pharmaceutical products of different formulas produced in the country in 1986, including 2,890 with generic names and 6,356 with trade names. By contrast, in 1984, there were only 6,371 types of pharmaceutical products produced.
A variety of pharmaceutical products are available on the market in various forms including tablets, syrups, ointments, powders, etc., each with different packages, although generically they are often the same or they have the same trademarks. Legally imported pharmaceutical products accounted for only about 2% of the domestic consumption because the government bans imports of products already produced in the country. Moreover, by offering investment opportunities to pharmaceutical companies, the government has promoted the development of the pharmaceutical industry, thereby reducing dependence on imports for products. Consequently, existing factories have met 95% of the country's pharmaceutical requirements and production capacity has increased considerably since 1978. Smuggled products and statistical discrepancies account for the difference. (See Table 5.)
Table 5
Indonesia's Pharmaceutical Production Capacity.
| Forms of Products | 1984/85 | 1985/86 | 1986/87 | 1987/88 |
| Tablets (mill.) | 9,653.0 | 8,753.0 | 11,593.4 | 23,244.5 |
| Capsules (mill.) | 1,304.0 | 996.8 | 1,588.1 | 4,575.6 |
| Syrup (mill.lt) | 8,078.6 | 9,100.0 | 9,750.1 | 6,117.0 |
| Inj.amps/vial (mill) | 141.3 | 142.1 | 156.0 | 237.4 |
| Powder (mill.kg) | 1.2 | 1.2 | 1.9 | 3.6 |
| Ointment (mill.kg) | .7 | .8 | 1.8 | n.a. |
|
Supp. (mill) |
.1 | .1 | .1 | .1 |
Source: G.P. Farmasi, 1988, Indonesian Association of Pharmaceutical Companies.
Ninety-five percent of basic (active) ingredients for Indonesia's pharmaceutical factories are imported. This is the case largely because the government (in a bid to reduce dependence on imports) has required foreign joint venture companies to produce at least one basic ingredient domestically. This regulation, however, could not be effectively enforced because only a few joint venture companies were able to comply with it.
A producer of basic ingredients faces many problems. For one thing, producing pharmaceutical basic ingredients requires high technology and expensive research facilities. In addition, the principal companies abroad may not be anxious to transfer their technology to their Indonesian partners. However, investors have shown genuine interest in building factories to produce antibiotic basic ingredients in Indonesia.
Imports
Legal imports account for only 2% of Indonesia's pharmaceutical products. By contrast, imports account for roughly 95% of the basic ingredients needed for the country's pharmaceutical industry. (See Table 6.)
Table 6
Imports of Pharmaceutical Product and
Basic (active) Ingredients 1985–1988.
| Year | Kgs | U.S. $ '000 |
| 1985 | 5,370,448 | 81,376 |
| 1986 | 5,916,155 | 90,687 |
| 1987 | 4,672,607 | 96,521 |
| 1988 | 4,517,995 | 99,032 |
Source: Central Bureau of Statistics, 1988.
Imported products and basic ingredients can be divided into the following groups: antibiotics, hormones, glycoside and organo therapeutic gland antisera, provitamins and vitamins. In 1986, imports of antibiotics totaled 992.7 tons, worth U.S. $39.6 million. In 1988 the import figures rose to 1,407.7 tons in volume, but dropped in value to U.S. $36.5 million. Imports of hormones, glycoside and organo therapeutics in 1986 totaled 655.1 tons worth U.S. $18 million and in 1988 the imports were recorded at 462.7 tons valued at U.S. $21.2 million. Vitamin and provitamin imports in 1988 were recorded at 931.8 tons, down from 1,191 tons in 1986, but the value rose from U.S. $12.5 million in 1986 to U.S. $15.4 million in 1988. (Source: Central Bureau of Statistics, 1988.)
In 1988, there were 157 companies who imported pharmaceutical basic ingredients, including 54 pharmaceutical companies, 42 pharmaceutical wholesalers, importers and 51 other organizations, such as government agencies, general importers and even animal feed traders. (See Table 7.)
Table 7
The Top Ten Pharmaceutical Importers.
| Rank | Importer | Value (U.S. $) |
| 1 | BKKBN Pusat | 8,801,517 |
| 2 | P.T. Jaya Bima Agung | 4,079,075 |
| 3 | P.T. Sandoz-Biochemie | 3,703,530 |
| 4 | P.T. Pharos Indonesia | 2,265,954 |
| 5 | P.T. Aneka Chemica | 2,219,343 |
| 6 | P.T. Charoen Pokphand | 1,913,641 |
| 7 | P.T. Enseval | 1,880,052 |
| 8 | P.T. Eurindo Combined | 1,429,364 |
| 9 | P.T. Beecham Pharmaceutical | 1,412,747 |
| 10 | P.T. Kimia Farma | 1,396,090 |
Source: P.T. Corinthian (CIC), 1990.
Basic ingredients for drugs are imported from West Germany, the U.S., Japan, the Netherlands, PR China, Italy, France, Australia and others. The biggest volume imported in 1988 came from the Netherlands, amounting to U.S. $9.7 million. The largest volume was for contraceptives, which were imported by Indonesia's Central Birth Control Coordinating Board (BKKBN) for its programs. Private companies also imported some contraceptives from the Netherlands, including NV PD Sudarpo Corp., P.T. Charoen Pokpand, P.T. Praja Farma and others.
The second biggest supplier of basic (active) ingredients for Indonesia was West Germany. In 1988, imports from this country amounted to U.S. $6.2 million, involving 47 companies including P.T. Hoechst, P.T. Merck Indonesia, P.T. Bayer Indonesia, P.T. Dumex Indonesia, P.T. Enseval and P.T. Kimia Farma.
The third biggest supplier in 1988 was the People's Republic of China (PRC), which exported by value U.S. $4.3 million of pharmaceutical products to Indonesia. Imports from the PRC involved 26 companies, including P.T. Menjangan Sakti, P.T. Ruskin Nusantara, P.T. Squibb, P.T. Pharos, P.T. Purusa Bhakti and Perum Indopharma. PRC imports included "traditional" Chinese remedies, as well as "modern" pharmaceuticals. The U.S. ranked eighth, importing pharmaceutical products worth U.S. $3.18 million to 27 companies in Indonesia.
Imports of pharmaceutical basic (active) ingredients showed a distinct trend to decline in volume, but to increase in value. Imports dropped in volume by 10% in 1986, but increased in value by 11% compared with 1985. In 1987, imports dropped again by 21.1% in volume, but increased by 6% in value from 1986. In 1988, imports dropped in volume by 3.4%, but rose in value by 2.6% from 1987.
For some products, such as antibiotic basic ingredients, however, the volume of imports continues to increase. Imports of antibiotic basic ingredients totaled 459,508 kg., worth U.S. $27.2 million in 1985 or U.S. $59.3 per kg. In 1988, imports totaled 1,175,346 kg., valued at U.S. $29.2 million or U.S. $24.8 per kg., a sharp drop in price per kg.
Import data for antibiotic basic ingredients indicate that pharmaceutical companies operating in the country have been engaged in keen competition, especially (foreign) joint venture companies versus (domestically owned) Indonesian firms. Over time, the "national" companies have become very price competitive with respect to antibiotics.
Exports
Despite heavy dependence on imports for basic raw materials, Indonesia has exported pharmaceutical products for a long time. In 1988, pharmaceutical product exports amounted to U.S. $26.3 million, up from U.S. $19.2 million in 1987 and U.S. $9.4 million in 1981. (See Table 8.)
Table 8
Indonesian Medicine Export Developments 1982–1988.
| Year | Kgs | U.S. $ |
| 1982 | 4,150,065 | 11,663,717 |
| 1983 | 3,764,069 | 13,928,838 |
| 1984 | 4,866,281 | 11,683,931 |
| 1985 | 5,647,620 | 15,391,736 |
| 1986 | 5,153,0,40 | 16,391,719 |
| 1987 | 6,842,545 | 19,176,893 |
| 1988 | 7,446,365 | 22,529,242 |
In 1988, exports of other products for medical and surgical purposes rose to U.S. $5.7 million from only U.S. $154,600 in 1987. Total export value of products made of local basic ingredients amounted to U.S. $21.5 million or 82% of the country's total exports of pharmaceutical products in 1988.
Exported products from Indonesia are mainly quinine and its derivatives as well as herbal medicines. Exports of quinine and its derivatives went mainly to West Germany and the U.S. Exports to West Germany in 1988 amounted to U.S. $3.4 million and to the U.S., U.S. $1.7 million. Quinine and its derivatives were also exported to Singapore, Spain, the U.K., Vietnam and Canada.
Exports of herbal medicine went mainly to Pakistan, Iran and Hong Kong. In 1988, herbal medicines exports to Pakistan alone amounted to U.S. $1.7 million, to Iran U.S. $1.2 million and Hong Kong U.S. $1.2 million. Exports in 1988 also went to other countries, including Bangladesh (U.S. $807,600), Singapore (U.S. $728,900), Malaysia, Libya, Taiwan, Yemen, the Netherlands and Germany.
Pricing Pharmaceutical Products
According to a regulation set by Indonesia's Food and Drug Supervision Directorate General, an apotik (dispensary) is to sell its product at a price 1.425 times the buying (net) price. (See the section, Distribution System of Pharmaceutical Products in Indonesia for a description of retail establishments distributing pharmaceuticals to the public.) A description of the price calculation system used in 1988 follows.
1. CIF (raw) price 100.0
Import duty 5.0
105.5
Value added tax (10%) 10.5
Handling cost 5.0
Land cost 120.5
2. Factory's processing cost
Processing cost 50.0
Auxiliary material cost 20.0
Packing 15.0
Marketing incl. advertising 40.0
3. Production Cost (1+2) 245.5
4. Factory's profit
(circa 30%) 73.7
5. Factory's Selling Price 319.2
(3+4)
6. Wholesaler's profit (20%) 63.8
383.0
7. Dispensary's buying price
Exploitation (10%) 38.3
421.3
Interest on capital
4x4% = 16% 67.4
488.7
Risk (10%) 48.9
537.6
Margin dispensary 40.7
8. Dispensary's selling price 577.9
Based on the CIF imports, the value of basic ingredients amounted to U.S. $124.7 million in 1988, and the market turnover of pharmaceutical products that year totaled 5.779 x U.S. $124.7 = U.S. $720.6 million. With a total population of 175.2 million in 1988, the country's per capita consumption of pharmaceutical products that year amounted to U.S. $4.1, far below those in Thailand, Malaysia, the Philippines, Singapore and South Korea, as seen in Table 9.
Table 9
Per Capita Consumption of Pharmaceutical Products.
1988 (U.S. $)
Thailand 4.1
Malaysia 5.1
Philippines 7.2
Singapore 10.0
South Korea 16.8
Australia 35.4
United States 44.1
Japan 81.7
Source: Indocommercial Business Report, 1991.
Market and Types of Products
The market for medicines depends largely on the pattern of the common diseases in a country. In tropical and developing countries, infectious diseases are the most common. For example, in 1987, 5.3 million Indonesians suffered from upper and lower respiratory tract diseases. Tooth and gum disease were second in prevalence, attacking 8.0% of the population. Skin and hypodermic diseases were third, accounting for 7.5% of cases. Eye and adnexa diseases amounted to 6.2% and cardiovascular diseases for 2.6%. This composition has not changed much during the last several years, with one exception: upper and lower respiratory tract cases have increased from 38.3% in 1987 to 41.0% in 1988 (possibly due to the considerable air pollution in all population centers).
Apotik sales of medicines reflect the characteristics of the pharmaceutical market in Indonesia. In 1988, antibiotic sales from apotiks accounted for 26.3% of the country's total sales of pharmaceuticals, topping other pharmaceuticals in sales that year. Respiratory system drugs are the second largest selling group of pharmaceuticals, sharing 12.6% of the country's total sales of pharmaceuticals in 1988. These two pharmaceuticals make up 38.9% of the country's total drug sales. This percentage corresponds to the pattern of common diseases in the country.
Drugs for the neuromuscular system held 11.6% of the pharmaceutical market, placing third in sales followed by vitamins and minerals, which shared 10.3% of the market. All in all, pharmaceutical sales in 1988 amounted to U.S. $720,641,000. Details are shown in Table 10.
Table 10
Market Share of Pharmaceutical Products by Sales–1988.
Figures in U.S. $ '000s
Pharmacological
Classification/ Market
Subclassification Sales Share(%)
Antibiotic 26.3
Penicillin (51.4%) 97,454.7
Macrolide (12.6%) 23,832.8
Chloramphenicol (9.6%) 18,220.0
Others (26.4%) 50,093.1
Total 189,600.6
Respiratory System 12.6
Cough & Cold Remedies (82.6%) 75,326.4
Antiasthmatic Preparations (15.7%) 14,266.7
Others (1.72%) 1,568.0
Total 91,161.0
Neuromuscular 11.6
Analgesic & Antipyretic (32.2%) 26,860.8
Antihistamine & Antiallergic (16.6%) 13,001.4
Antirheumatic (10.4%) 8,694.7
Anti-inflammatory Enzymes (8.8%) 7,350.7
Others (32.0%) 26,734.7
Total 83,522.3
Vitamins & Minerals 10.3
Vitamin B Complex (24.1%) 17,915.8
Multivitamin w/Mineral (22.3%) 16,614.3
Appetite Stimulant (12.3%) 9,132.6
Antianaemics (10.7%) 7,935.3
Others (30.6%) 22,772.1
Total 74,370.1
Dermatological Drugs 9.4
Anti-infective w/Corticosteroid (33.9%) 23,027.6
Fungicides & Antiparasites (15.8%) 10,707.7
Topical Corticosteroid (15.4%) 10,469.6
Anti-infective (10.7%) 7,299.4
Others (24.3%) 16,524.1
Total 68,028.5
Other Chemotherapeutics 6.1
Other Antituberculous Agents (41.6%) 13,238.4
Anti Diarrheals (18.0%) 7,895.3
Sulphanomides (12.1%) 5,310.3
Others ??
Total 43,887.0
Cardiovascular System & Diuretic 42,661.9 5.9
Alimentary System 41,220.7 5.7
Nutritive Drugs 22,988.4 3.2
Hormone Drugs 15,998.2 2.2
Others 47,418.2 6.6
TOTAL 720,641
Source: P.T. Corinthian (CIC), 1990.
Ranks of Pharmaceutical Manufacturers by Sales
In 1989, forty foreign joint venture companies operating in Indonesia dominated 57.7% of the domestic market, according to apotik sales records. The 245 Indonesian companies captured 42.3% of the market. Foreign companies are expected to continue to hold a larger market share than domestic firms in the near future for two reasons. First, Indonesian companies do not hold product patents, and while foreign patents are not always honored in Indonesia, the risk of legal complications may constrain some domestic firms to wait until the patent has expired before producing the same product. Second, consumers, including medical doctors, may have more confidence in the products of the foreign companies as compared to those of domestic firms.
There are some local companies, however, that have managed remarkable gains in marketing, such as P.T. Sanbe Farma. In 1988, it outranked all other producers. P.T. Kalbe Farma, which topped the list in 1976 and 1982/1983, fell to third place in 1989. P.T. New Interbat jumped from twenty-fifth in 1976 to sixth in 1982/1983, but slipped again to eleventh in 1989. P.T .Praja Farma (P.T. Prafa) climbed from sixty-second in 1976 to thirty-sixth in 1982/1983 and to seventeenth in 1989. P.T. Pharos Indonesia slipped from thirty-ninth in 1976 to forty-fifth in 1982/1983, but improved its position by climbing to twenty-first in 1989. P.T. Kimia Farma has been on the decline since 1976, when it fell from second to nineteenth in 1982/1983 and to twenty-second in 1989.
Among the foreign joint venture companies, P.T. Beecham jumped from seventeenth in 1976 to tenth in 1982/1983, and to second in 1989. P.T. Merck, which ranked second in 1976, fell to fourth in 1982/1983 and remained in the same position until 1989. P.T. Rhone Poulenc ranked eleventh in 1976 and managed to climb to third in 1982/1983; however, it slipped to fifth in 1989. (See Table 11.)
Table 11
List of The Top Twenty-Five Pharmaceutical Companies by Sales–1989.
|
Rank |
Companies |
Market Proportion (%) |
Rank in 1983 |
| 1 | Sanbe Farma | 3.9 | 22 |
| 2 | Beecham | 3.8 | 10 |
| 3 | Kalbe Farma | 3.7 | 1 |
| 4 | Merck | 3.1 | 4 |
| 5 | Rhone Poulenc | 3.0 | 3 |
| 6 | Squibb | 2.9 | 9 |
| 7 | Burroughs Wellcome | 2.7 | 48 |
| 8 | Ciba-Geigy | 2.7 | 15 |
| 9 | Schering USA | 2.6 | 18 |
| 10 | Parke-Davis | 2.5 | - |
| 11 | New Interbat | 2.4 | 6 |
| 12 | Mead Johnson | 2.3 | 14 |
| 13 | Boehringer Ingerlheim | 2.2 | 13 |
| 14 | Hoechst | 2.2 | 8 |
| 15 | Sandoz Biochemie | 2.0 | 23 |
| 16 | Bayer | 1.9 | 20 |
| 17 | Praja Farma | 1.8 | 36 |
| 18 | Roche | 1.8 | 5 |
| 19 | Abbott | 1.8 | 11 |
| 20 | Janssen | 1.7 | |
| 21 | Pharos Indonesia | 1.7 | 45 |
| 22 | Kimia Farma | 1.7 | 19 |
| 23 | Upjohn | 1.6 | 26 |
| 24 | Dumex | 1.6 | 21 |
| 25 | Pfizer | 1.5 | 7 |
Source: P.T. Corinthian (CIC), 1990.
Distribution System of Pharmaceutical Products in Indonesia
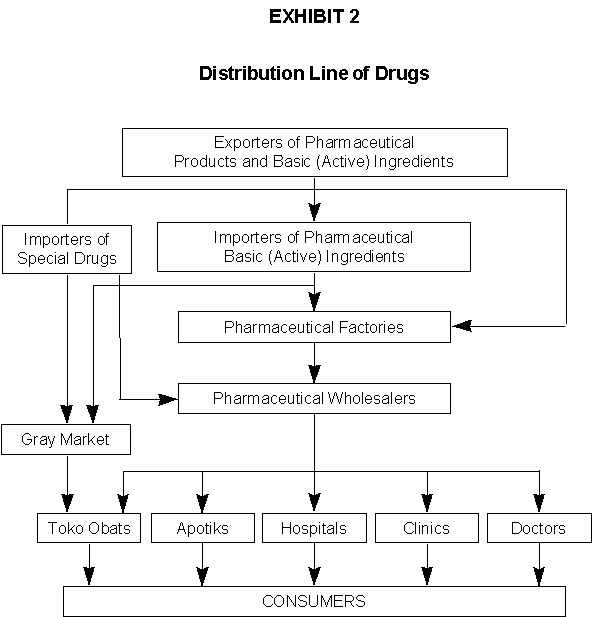
General System of Distribution
To get a license for a new product, a pharmaceutical company must apply to the Indonesian Department of Health. The procedure takes two months to a year, depending on the product. Companies who apply for a license eventually get one. The main problem is the government bureaucracy, which is sometimes very inefficient. Sometimes "facilitating fees" may have to be paid to officials to get the license easier and on time. This practice is common in Indonesia, although, of course, it is illegal. Foreign companies with U.S. parents face the special jeopardy of the (U.S.) Foreign Corrupt Practices Act in this context.
In Indonesia, factories are not allowed to sell their products directly to the consumers or retailers; they must go through wholesalers. There are two major types of retail establishments that distribute pharmaceutical products to the public. Apotik is the Indonesian term for pharmacy, apothecary or dispensary. Apotiks sell prescription drugs and a small number of OTC drugs. Most apotiks are agents of particular pharmaceutical firms. They are highly regulated by the government, and must be managed by certified pharmacists. Toko obats, meaning medicine shops, sell OTC drugs, along with some fakes and illegally imported drugs from the gray market. The products sold by toko obats are usually cheaper than those sold by apotiks.
The distribution pattern and the state of the pharmaceutical industry in general is characterized by two distinct government policies that are partially conflicting. On one hand, the government attempts to maximize national capabilities including ownership of pharmaceutical production and distribution, which enable the country to be "self-sufficient" in providing drugs to the people at minimum cost. On the other hand, the Indonesian government, like many others, has been concerned with the safety of drugs offered. Thus, in order to help the "local" pharmaceutical industry to grow and, at the same time, control the quality of drugs in the market, Indonesia banned imports of most medicinal drugs in 1976. Imports accounted for only 2% of the country's consumption of pharmaceuticals by the end of the 1980s. Local producers, therefore, do not have to fear competition from imported products.
However, many importers have been known to resort to illegal operations since the government ban on imports. Some wholesale companies, which previously operated as importers, have turned to illicit trade, such as smuggling and/or producing unauthorized imitations in order to survive.
Products smuggled from Taiwan, Singapore or Hong Kong are not all fakes, but name brand products that are cheaper to import because production costs in those countries are lower. Most of the products sold via the gray market are nonregulated OTC drugs, which are sold by toko obats and wholesalers. Apotiks and hospitals do not buy gray market products. The government has taken some measures against companies found engaged in illegal activities. On the other hand, the government has encouraged previous importers to operate as distributors.
Although Indonesia has officially established a system of Patent Rights, there are still lots of fake products in the pharmaceutical market. Some "look alike" brands are still legally permitted, especially those produced by local companies. These products are cheaper than the "originals." There are also some imported fakes from Taiwan and Hong Kong. Most of them are cheaper, and sold by toko obats that have to comply with few regulations in this regard. By contrast, most apotiks do not sell fakes, because there are regulations against selling imitation products. Apotiks caught violating these regulations may be fined or even closed by the government.
Producers are largely required to leave the promotion and distribution tasks to wholesale companies. Increasing requirements for wholesale companies to serve as both distributors and promotional agents has made the distribution business more interesting. It is financially attractive because of the relatively low cost, since most of the costs are paid by the pharmaceutical producers. As the major link between the pharmaceutical industry and the market, the business is relatively safe, due to the continuing demand for drugs. A growing number of companies have been attracted into this line of business. In 1969, there were only 500 companies operating as distributors. In 1988 the figure increased to 1,081. Over half (54.6%) of these distributors operate in Java; Jakarta alone has 252 such firms.
According to one estimate, 50% of the pharmaceuticals produced in Indonesia are distributed through apotiks, 30% are distributed to toko obats and the remaining 20% are distributed directly by practicing physicians and hospitals.
Therefore, apotiks play an important role in Indonesia's pharmaceutical industry. In 1988, there were 2,211 apotiks registered in the country compared to 1,672 in 1985. While apotiks are located all over the country, most are on Java. In 1985, there were 1,150 apotiks on Java, or 68.8% of the total number in the country. Jakarta alone had 318. In 1988, the number of apotiks increased by 92.2 % compared with 1985. Java remained on top with 68%.
The role of toko obats is also important. Toko obats sell pharmaceuticals at relatively cheaper prices, often 10% to 20% less than apotiks. Many people therefore, buy from toko obats rather than from apotiks. The number of toko obats operating in Indonesia has also increased. Many of them are not properly registered, although the government has tightened licensing procedures. In 1985 there were 1,201 registered toko obats; in 1988 the figure rose to approximately 2000.
As is the case with wholesale companies and apotiks, Java has the largest number of toko obats. In 1988, there were 1,012 toko obats on Java, accounting for 50.5% of the total number in the country. Toko obats, however, are spread more evenly than apotiks. Effectively, toko obats serve in the role of apotiks in small towns and rural areas.
In addition to OTC drugs, stronger drugs are also available at the toko obats outside metropolitan areas. In these locations, toko obats act like apotiks, and, on occasion, strong drugs are even sold by cigarette retailers on the street. See Exhibit 2.)
Pharmaceutical Sales Promotion and Price Forming
Pharmaceutical factories advertise their nonethical products through the media such as newspapers, magazines, radio and billboards. Ethical products are advertised through medical and pharmaceutical magazines, such as the Index of Indonesia's Medical Services (IIMS), Informasi Spesialis Obat (Indonesia) (ISO), and especially through promotional seminars. Some producers continue to hire "detailers" (pharmaceutical salespeople) to promote their products directly to practicing physicians. Such detailing is not always effective, however, because most MDs do not like to meet detailers in person. Instead, doctors tell their nurses to pick up the samples. Then the doctors contact the detailer later by phone.
The government health authorities also discourage the use of detailers because they are considered to be "disturbing the established order." Detailers are no longer allowed to go into hospitals, especially government hospitals. Consequently, most companies now conduct seminars and workshops to introduce new products, rather than use detailers. At these seminars, companies present papers and lab results in conjunction with marketing their products, as is the practice in industrialized countries. Also, as in the developed countries, manufacturers promote products containing the same active ingredients under different names and labels, in order to segment the market. Promotion costs are high and constitute an important factor in price calculations. Some people in the industry figure that promotional costs account for up to 30% of the price.
The long distribution line is also a factor in pricing. Under the current system in Indonesia, pharmaceutical factories distribute their products through a wholesaler in order to service apotiks, toko obats and hospitals.
The Indonesian Association of Pharmaceutical Companies (GPFI) plays an important role in setting the price of pharmaceutical products for the consumer. Based on the old price structure, an apotik sells its pharmaceutical products at a price 175% above the factory's selling price with apotiks enjoying a 30% discount and hospitals/health centers 20%. Some producers, though, offer higher discounts in order to beat the competition in these markets.
In Indonesia there are only 3000 apotiks and toko obats to service the country's 180 million people. Complicating the matter further, most Indonesians live in rural areas, whereas most apotiks and toko obats are located in cities. Consequently, rural people obtain drugs from government-run health centers, called Puskesmas and Pos Yandu. A Puskesmas is a subdistrict public health center served by two or three "junior" doctors and nurses. It is not well-equipped and cannot treat severe illness, but provides adequate services for general sickness and first aid before patients can be treated at a hospital. A Pos Yandu is a rural area public service center that functions as a multipurpose service and firstaid post. Its’ staff typically includes a nurse and several social workers, but no doctor. It also serves as an information center for government family planning, nutrition and welfare programs.
Characteristics of the Indonesian Health Care Delivery System
Only 17,000 of the 22,000-27,000 MDs in Indonesia are actually practicing. A medical career in Indonesia is not considered attractive any more. Some MDs teach at medical schools but do not practice, others join the pharmaceutical industry, and some become consultants. However, most of the MDs who do not practice are women. One reason so many female MDs do not practice is the government stipulation requiring all graduating medical students to serve in a rural area for two to three years before they are allowed to set up a regular practice. Many female MDs come from upper class families and do not need to work for financial reasons, especially after marrying. Consequently, they do not leave their families to work in rural areas and, therefore, they do not practice medicine.
On the demand side, only 2% of Indonesia's GDP goes to health care (in the U.S. the corresponding number is over 8%). At the same time, most low-to-middle class Indonesians cannot afford health insurance, and the government does not have the money to provide insurance. Most upper class Indonesians consider health insurance unnecessary because they can cover their medical expenses by themselves. Only some large companies insure their employees, mostly against work-related accidents.
Projected Consumption
The improving economic situation in Indonesia will impact the pharmaceutical market significantly as the buying power of the people increases. Indicators of rising consumption of medicines in the future are: (1) an improving economy; (2) a population growth of 2% to 2.3% per year; and (3) an increasing trend in per capita spending on non-food products from 36.8% in 1984 to 38.7% in 1987. It is estimated that consumption of medicines will increase by 4% per year in the 1990s. In 1990, the consumption figures rose to U.S. $778.2 million. (See Table 12.)
Table 12
Projection of Drug Consumption.
1991–1993
Year U.S. $'000
1991 807,116
1992 835,941
1993 864,766
Source: P.T. Corinthian (CIC), 1990.
In the next five years, it is believed that the pattern of the common diseases will remain virtually the same. There will be new diseases, but the number of cases is not expected to affect the traditional pattern in significant ways.
It is estimated that the domestic consumption of antibiotics in 1991 will amount to U.S. $204.8 million based on 1988 dispensary sales figures. Consumption of medicines for the respiratory system will be the second largest at U.S. $98.2 million. The third largest seller is expected to be for the neuromuscular system (U.S. $90.2 million). (Source: P.T. Corinthian (CIC), 1990.)
The State of the Pharmaceutical Industry in Indonesia: Summary
The pharmaceutical industry in Indonesia is one of the business sectors that has recorded steady growth. As evidence, it appears that there is always an investor eager to rescue any failing pharmaceutical company. Moreover, there are 285 pharmaceutical factories operating in Indonesia, and that number is expected to grow as the government continues to encourage development. Meanwhile, the market for pharmaceutical products in the country has increased, with antibiotics continuing to have the largest market share.
The large market demand for pharmaceutical products, however, has not encouraged investment in basic ingredient manufacturing. About 95% of the basic ingredients are supplied through imports; only 5% come from local sources. Despite government encouragement, no domestic investor has been interested in venturing into the pharmaceutical basic ingredients industry. Technological and marketing problems, coupled with the difficulties of obtaining manufacturing licenses from foreign principals, are among the obstacles discouraging investors from committing to this business.
Foreign joint venture companies receive basic ingredient supplies from their parent companies abroad and, without government requirements, would most likely eliminate their costly chemical plants in Indonesia. Imports are expected to fill the basic ingredient supply in the future.
In 1988, the forty foreign joint ventures operating in Indonesia together shared 60% of the pharmaceutical products market. The 245 domestic companies held a 40% share. Some of the domestic companies including Sanbe, Kalbe Farma, New Interbat and Praja Farma have grown to secure a strong market share in competition with foreign companies. It is expected that despite increasing demand, market competition will remain severe and foreign companies especially will lose market share to aggressive "national" companies protected by various government policies. Producers will have to closely follow market developments before considering new products. A profile of the ten largest pharmaceutical companies operating in Indonesia is provided in Table 13.
Table 13
Profiles of The Top Ten Pharmaceutical Competitors in Indonesia.
Ranked by sales as of 1989.
1. P.T. Sanbe Farma
Established: 1973
Status: Private National Company
Total Investment: a. Own capital Rp. 2,000 million
b. Loan capital Rp. 2,000 million
c. Total Rp. 4,000 million
Production Capacity Real Production-1988
Capsule 16,651,000 pcs pa 1,198,400 pcs pa
Tablet 21,604,000 pcs pa 2,258,900 pcs pa
Syrup 96,800 lt pa 74,463 lt pa
Injectable 8,785,000 vial/amp pa 580,200 vial/amp pa
Cream 1.3 ton pa 0.65 ton pa
Powder 6 ton pa 4.08 ton pa
Total Employees: 350
2. P.T. Beecham Pharmaceutical Indonesia
Established: 1979
Status: Foreign Investment company
Total Investment: U.S. $4,519,000
Production Capacity Real Production-1988
Tablet 45,350,000 pcs pa 2,600,000 pcs pa
Capsule 80,000,000 pcs pa 17,000,000 pcs pa
Syrup 3,770,000 lt pa 350,000 lt pa
Ointment 3,200,000 pa N.A.
3. P.T. Kalbe Farma
Established: 1966
Status: Private National & Domestic Investment Company
Total Investment: Rp. 12,000 million
Production Capacity Real Production-1988
Capsule 88,000,000 pcs pa 68,000,000 pcs pa
Tablet 469,000,000 pcs pa 375,000,000 pcs pa
Syrup 461,000 lt pa 424,500 lt pa
Injectable 9,500,000 vial/amp pa 5,200,000 vial/amp pa
Cream 30 ton pa 31 ton pa
Powder 43 ton pa 13 ton pa
Total Employees: 1500
4. P.T. Merck Indonesia
Established: 1970
Status: Foreign Investment Company
Total Investment: U.S. $11,637,000
Total Employees: 294
5. P.T. Rhone Poulenc Indonesia Pharma
Established: 1971 as P.T. Rhodia Indonesia
1980 as P.T. Rhone Poulenc Indonesia Pharma
Status: Foreign Investment Company
Total Investment: U.S. $6,211,900
Production Capacity Real Production-1988
Capsule 2,500,000 pcs pa 1,393,000 pcs pa
Tablet 40,000,000 pcs pa 35,432,444 pcs pa
Syrup 100,000 lt pa 63,850 lt pa
Cream 18.75 ton pa 5.3 ton pa
Total Employees: 300
6. P.T. Squibb Indonesia
Established: 1970
Status: Foreign Investment Company
Total Investment : U.S. $9,832,000
Production Capacity Real Production-1988
Capsule 18,000,000 pcs pa 6,292,500 pcs pa
Tablet 125,000,000 pcs pa 51,356,500 pcs pa
Syrup 90,000 lt pa 57,003 lt pa
Injectable 2,000,000 vial/amp pa 58,000 vial/amp pa
Cream 30 ton pa 20 ton pa
Powder 14 ton pa 3.6 ton pa
Total Employees: 326
7. P.T. Burroughs Wellcome Indonesia
Established: 1982 as P.T. Wellcome Indonesia
1983 as P.T. Burroughs Wellcome Indonesia
Status: Foreign Investment Company
Total Investment: U.S. $3,500,000
Production Capacity Real Production-1988
Tablet 50,000,000 pcs pa 13,800,000 pcs pa
Syrup 84,000 lt pa 18,700 lt pa
Total Employees: 217
8. P.T. Ciba-Geigy Pharma Indonesia
Established: 1969 as P.T. Ciba Indonesia
1971 as P.T. Ciba-Geigy Pharma Indonesia
Status: Foreign Investment Company
Total Investment : a. Equity capital U.S. $3,000 million
b. Loan capital U.S. $1,000 million
c. Total U.S. $4,000 million
Production Capacity Real Production-1988
Capsule 112,000,000 pcs pa 33,000,000 pcs pa
Tablet 240,000,000 pcs pa 78,000,000 pcs pa
Syrup 21,000 lt pa 4,168 lt pa
Cream 21 ton pa 1.75 ton pa
Powder 70 ton pa N.A.
Total Employees: 200
9. P.T. Schering Indonesia
Established: 1970
Status: Foreign Investment Company
Total Investment: U.S. $2,892,300
Production Capacity Real Production-1988
Capsule 7,700,000 pcs pa 1,720,234 pcs pa
Tablet 82,700,000 pcs pa 72,099,109 pcs pa
Syrup 102,000 lt pa 60,700 lt pa
Cream 8.9 ton pa 8.5 ton pa
Total Employees: 350
10. P.T. Warner-Lambert Indonesia
Established: 1970
Status: Foreign Investment Company
Total Investment: U.S. $4,000,000
Shareholders: Parke-Davis & Co., USA
Warner-Lambert Co. AG, Switzerland
P.T. Loho Industry Pharmasi, Indonesia
Production Capacity Real Production-1988
Capsule 116,000,000 pcs pa 29,000,000 pcs pa
Tablet 180,000,000 pcs pa 25,000,000 pcs pa
Syrup 2,200,000 lt pa 2,200,000 lt pa
Cream 72 ton pa 2.5 ton pa
Total Employees: 176
Source: P.T. Corinthia (CIC), 1990.
Medicines account for 30% to 40% of health care costs in Indonesia. With most people having little buying power, per capita consumption of medicines in Indonesia is very low, at about U.S. $4.1 in 1988. Because of the low income level the government encourages the use of generics, particularly by Puskesmas. Most people, especially in rural areas, cannot afford to buy products from foreign manufacturers, so they simply purchase the generic products at cheaper prices.
For 1989/1990, the budget for medical supplies amounted to Rp. 104,204 million, consisting of Rp. 13,125 million for medical supplies to all hospitals, Rp. 3,648 million for supplies to the main units of the health ministry. With a population of 178 million, the per capita subsidy is about Rp. 585 per year.
Since 1978 the government has sought to provide cheap, but good quality, medicines for the public through the INPRES and health insurance programs by supplying generic medicines. The government has a national policy to popularize generic medicines in the country. At the same time, these government efforts are not always effective because sometimes consumers believe that inexpensive medicines are of low quality, and doctors do not prescribe generic medicines for their patients. However, in 1989, the health minister obliged medical doctors to prescribe or use generic medicines for their patients at government health centers by imposing the following regulations:
The Health Ministry has provided Rp. 1.8 billion to finance these changes. Funds will be used to publish guide books and for promotional efforts through television, radio and medical doctors. Despite this government effort, some industry insiders estimate that generic medicines will share no more than 30% of the domestic market in the future and therefore will not pose a strong threat to patent products.
One problem hampering the program in popularizing generic medicines includes stock shortages and the fact that the three state companies designated to produce these drugs failed to meet the demand. Starting in 1990, however, the government allowed privately-owned pharmaceutical companies to produce generic medicines. It is believed that participation of the private sector in producing generic medicines will overcome these shortages and also develop the pharmaceutical industry.
General Issues Shaping the Regulatory Environment of the Pharmaceutical Industry
It must be recognized that the pharmaceutical industry worldwide is subject to extensive government regulation. First of all, it is generally accepted that pharmaceuticals require special government supervision because of the inability of consumers to assess effectiveness and potentially damaging side effects. It is not that there are always clear answers to these issues. The assessment of such effects is often controversial even among medical and pharmaceutical specialists, especially when weighted against the cost of forgoing potential remedies. Second, in addition to this aspect of public safety, there is the issue related to health care as a highly politicized service. In virtually all countries, government plays a disproportionate role in laying out the conditions of health care delivery, including the monopolization of the entire delivery system. For example, in some countries, such as the U.K., Canada or various socialist countries, government is essentially the sole provider of health care. In other countries, the government only administers medical services for the poor. Beyond that, governments oversee or provide health insurance and thereby influence marketing for health care services, including pharmaceuticals. With respect to the first issue, the dilemma involves balancing public safety against the development of better pharmaceuticals. With respect to the second issue, the dilemma is more complex, since theoretically the demand for health services is virtually unlimited. Consequently, society has to choose where and how to allocate the proportion of total resources devoted to health care. For example, a wealthy society like the U.S. commits over 8% of its GDP to health care; whereas, in Indonesia the proportion is 2% or less and only 10% to 15% of the population have any health insurance at all.
There is a third issue that has a unique north-south dimension. Virtually all pharmaceuticals are discovered and tested in industrialized countries because of the capital- and science-intensive process required. Pharmaceutical research and development requires a handful of brilliant scientists (which many LDCs have), but also small armies of skilled chemists, lab technicians, skilled plumbers, and electricians (which LDCs do not have in ample supply).
For this reason, LDCs with typically large, low-income populations have a constant incentive to make available the results of pharmaceutical research at only a marginal cost, while refusing to grant patent protection and other privileges necessary to continue the process of pharmaceutical innovation, which must go on somewhere.